Nanoparticle-assisted optical tethering of endosomes reveals the cooperative function of dyneins in retrograde axonal transport
- PMID: 26656461
- PMCID: PMC4674899
- DOI: 10.1038/srep18059
Nanoparticle-assisted optical tethering of endosomes reveals the cooperative function of dyneins in retrograde axonal transport
Abstract
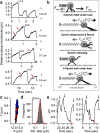
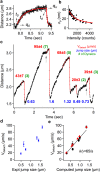
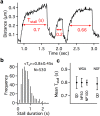
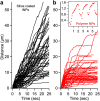
Dynein-dependent transport of organelles from the axon terminals to the cell bodies is essential to the survival and function of neurons. However, quantitative knowledge of dyneins on axonal organelles and their collective function during this long-distance transport is lacking because current technologies to do such measurements are not applicable to neurons. Here, we report a new method termed nanoparticle-assisted optical tethering of endosomes (NOTE) that made it possible to study the cooperative mechanics of dyneins on retrograde axonal endosomes in live neurons. In this method, the opposing force from an elastic tether causes the endosomes to gradually stall under load and detach with a recoil velocity proportional to the dynein forces. These recoil velocities reveal that the axonal endosomes, despite their small size, can recruit up to 7 dyneins that function as independent mechanical units stochastically sharing load, which is vital for robust retrograde axonal transport. This study shows that NOTE, which relies on controlled generation of reactive oxygen species, is a viable method to manipulate small cellular cargos that are beyond the reach of current technology.
Figures


Similar articles
-
Snapin recruits dynein to BDNF-TrkB signaling endosomes for retrograde axonal transport and is essential for dendrite growth of cortical neurons.Cell Rep. 2012 Jul 26;2(1):42-51. doi: 10.1016/j.celrep.2012.06.010. Epub 2012 Jul 12. Cell Rep. 2012. PMID: 22840395 Free PMC article.
-
Axonal autophagosomes recruit dynein for retrograde transport through fusion with late endosomes.J Cell Biol. 2015 May 11;209(3):377-86. doi: 10.1083/jcb.201412046. Epub 2015 May 4. J Cell Biol. 2015. PMID: 25940348 Free PMC article.
-
Dynamic Clustering of Dyneins on Axonal Endosomes: Evidence from High-Speed Darkfield Imaging.Biophys J. 2018 Jul 17;115(2):230-241. doi: 10.1016/j.bpj.2018.05.026. Epub 2018 Jun 19. Biophys J. 2018. PMID: 29933888 Free PMC article.
-
Action in the axon: generation and transport of signaling endosomes.Curr Opin Neurobiol. 2008 Jun;18(3):270-5. doi: 10.1016/j.conb.2008.08.005. Curr Opin Neurobiol. 2008. PMID: 18778772 Free PMC article. Review.
-
Retrograde signaling via axonal transport through signaling endosomes.J Pharmacol Sci. 2019 Oct;141(2):91-96. doi: 10.1016/j.jphs.2019.10.001. Epub 2019 Oct 17. J Pharmacol Sci. 2019. PMID: 31679963 Review.
Cited by
-
Detection of Velocity and Diffusion Coefficient Change Points in Single-Particle Trajectories.Biophys J. 2018 Jul 17;115(2):217-229. doi: 10.1016/j.bpj.2017.11.008. Epub 2017 Dec 11. Biophys J. 2018. PMID: 29241585 Free PMC article.
-
Real-Time Tracking of Vesicles in Living Cells Reveals That Tau-Hyperphosphorylation Suppresses Unidirectional Transport by Motor Proteins.Chem Biomed Imaging. 2024 Apr 23;2(5):362-373. doi: 10.1021/cbmi.4c00016. eCollection 2024 May 27. Chem Biomed Imaging. 2024. PMID: 39474119 Free PMC article.
-
Neuronal delivery of nanoparticles via nerve fibres in the skin.Sci Rep. 2021 Jan 28;11(1):2566. doi: 10.1038/s41598-021-81995-x. Sci Rep. 2021. PMID: 33510229 Free PMC article.
-
Neural Tracing Protein-Functionalized Nanoparticles Capable of Fast Retrograde Axonal Transport in Live Neurons.Small. 2024 Sep;20(39):e2311921. doi: 10.1002/smll.202311921. Epub 2024 Apr 22. Small. 2024. PMID: 38647340 Free PMC article.
-
Effects of dynein inhibitor on the number of motor proteins transporting synaptic cargos.Biophys J. 2021 May 4;120(9):1605-1614. doi: 10.1016/j.bpj.2021.02.018. Epub 2021 Feb 20. Biophys J. 2021. PMID: 33617835 Free PMC article.
References
-
- von Bartheld C. S. Axonal transport and neuronal transcytosis of trophic factors, tracers, and pathogens. J Neurobiol 58, 295–314 (2004). - PubMed
-
- Chowdary P. D., Che D. L. & Cui B. Neurotrophin Signaling via Long-Distance Axonal Transport. Annual Review of Physical Chemistry, Vol 63 63, 571–594 (2012). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

