The Basic Domain of Herpes Simplex Virus 1 pUS9 Recruits Kinesin-1 To Facilitate Egress from Neurons
- PMID: 26656703
- PMCID: PMC4733978
- DOI: 10.1128/JVI.03041-15
The Basic Domain of Herpes Simplex Virus 1 pUS9 Recruits Kinesin-1 To Facilitate Egress from Neurons
Abstract
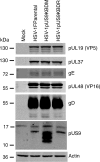
The alphaherpesviral envelope protein pUS9 has been shown to play a role in the anterograde axonal transport of herpes simplex virus 1 (HSV-1), yet the molecular mechanism is unknown. To address this, we used an in vitro pulldown assay to define a series of five arginine residues within the conserved pUS9 basic domain that were essential for binding the molecular motor kinesin-1. The mutation of these pUS9 arginine residues to asparagine blocked the binding of both recombinant and native kinesin-1. We next generated HSV-1 with the same pUS9 arginine residues mutated to asparagine (HSV-1pUS9KBDM) and then restored them being to arginine (HSV-1pUS9KBDR). The two mutated viruses were analyzed initially in a zosteriform model of recurrent cutaneous infection. The primary skin lesion scores were identical in severity and kinetics, and there were no differences in viral load at dorsal root ganglionic (DRG) neurons at day 4 postinfection (p.i.) for both viruses. In contrast, HSV-1pUS9KBDM showed a partial reduction in secondary skin lesions at day 8 p.i. compared to the level for HSV-1pUS9KBDR. The use of rat DRG neuronal cultures in a microfluidic chamber system showed both a reduction in anterograde axonal transport and spread from axons to nonneuronal cells for HSV-1pUS9KBDM. Therefore, the basic domain of pUS9 contributes to anterograde axonal transport and spread of HSV-1 from neurons to the skin through recruitment of kinesin-1.
Importance: Herpes simplex virus 1 and 2 cause genital herpes, blindness, encephalitis, and occasionally neonatal deaths. There is also increasing evidence that sexually transmitted genital herpes increases HIV acquisition, and the reactivation of HSV increases HIV replication and transmission. New antiviral strategies are required to control resistant viruses and to block HSV spread, thereby reducing HIV acquisition and transmission. These aims will be facilitated through understanding how HSV is transported down nerves and into skin. In this study, we have defined how a key viral protein plays a role in both axonal transport and spread of the virus from nerve cells to the skin.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
Dual Role of Herpes Simplex Virus 1 pUS9 in Virus Anterograde Axonal Transport and Final Assembly in Growth Cones in Distal Axons.J Virol. 2015 Dec 23;90(5):2653-63. doi: 10.1128/JVI.03023-15. J Virol. 2015. PMID: 26699637 Free PMC article.
-
Herpes Simplex Virus gE/gI and US9 Promote both Envelopment and Sorting of Virus Particles in the Cytoplasm of Neurons, Two Processes That Precede Anterograde Transport in Axons.J Virol. 2017 May 12;91(11):e00050-17. doi: 10.1128/JVI.00050-17. Print 2017 Jun 1. J Virol. 2017. PMID: 28331094 Free PMC article.
-
Anterograde Viral Tracer Herpes Simplex Virus 1 Strain H129 Transports Primarily as Capsids in Cortical Neuron Axons.J Virol. 2020 Mar 31;94(8):e01957-19. doi: 10.1128/JVI.01957-19. Print 2020 Mar 31. J Virol. 2020. PMID: 31969440 Free PMC article.
-
Infection and Transport of Herpes Simplex Virus Type 1 in Neurons: Role of the Cytoskeleton.Viruses. 2018 Feb 23;10(2):92. doi: 10.3390/v10020092. Viruses. 2018. PMID: 29473915 Free PMC article. Review.
-
Transport and egress of herpes simplex virus in neurons.Rev Med Virol. 2008 Jan-Feb;18(1):35-51. doi: 10.1002/rmv.560. Rev Med Virol. 2008. PMID: 17992661 Review.
Cited by
-
Molecular association of herpes simplex virus type 1 glycoprotein E with membrane protein Us9.Arch Virol. 2016 Nov;161(11):3203-13. doi: 10.1007/s00705-016-3028-z. Epub 2016 Aug 27. Arch Virol. 2016. PMID: 27568015 Free PMC article.
-
Manipulation of Host Microtubule Networks by Viral Microtubule-Associated Proteins.Viruses. 2022 May 6;14(5):979. doi: 10.3390/v14050979. Viruses. 2022. PMID: 35632720 Free PMC article. Review.
-
Herpes simplex virus-1 utilizes the host actin cytoskeleton for its release from axonal growth cones.PLoS Pathog. 2022 Jan 24;18(1):e1010264. doi: 10.1371/journal.ppat.1010264. eCollection 2022 Jan. PLoS Pathog. 2022. PMID: 35073379 Free PMC article.
-
Dual Role of Herpes Simplex Virus 1 pUS9 in Virus Anterograde Axonal Transport and Final Assembly in Growth Cones in Distal Axons.J Virol. 2015 Dec 23;90(5):2653-63. doi: 10.1128/JVI.03023-15. J Virol. 2015. PMID: 26699637 Free PMC article.
-
A Human Stem Cell-Derived Neurosensory-Epithelial Circuitry on a Chip to Model Herpes Simplex Virus Reactivation.Biomedicines. 2022 Aug 24;10(9):2068. doi: 10.3390/biomedicines10092068. Biomedicines. 2022. PMID: 36140168 Free PMC article.

