Comparative Mutagenesis of Pseudorabies Virus and Epstein-Barr Virus gH Identifies a Structural Determinant within Domain III of gH Required for Surface Expression and Entry Function
- PMID: 26656711
- PMCID: PMC4810733
- DOI: 10.1128/JVI.03032-15
Comparative Mutagenesis of Pseudorabies Virus and Epstein-Barr Virus gH Identifies a Structural Determinant within Domain III of gH Required for Surface Expression and Entry Function
Abstract
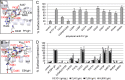
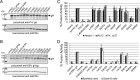
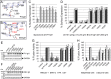
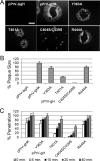
Herpesviruses infect cells using the conserved core fusion machinery composed of glycoprotein B (gB) and gH/gL. The gH/gL complex plays an essential but still poorly characterized role in membrane fusion and cell tropism. Our previous studies demonstrated that the conserved disulfide bond (DB) C278/C335 in domain II (D-II) of Epstein-Barr virus (EBV) gH has an epithelial cell-specific function, whereas the interface of D-II/D-III is involved in formation of the B cell entry complex by binding to gp42. To extend these studies, we compared gH of the alphaherpesvirus pseudorabies virus (PrV) with gH of the gammaherpesvirus EBV to identify functionally equivalent regions critical for gH function during entry. We identified several conserved amino acids surrounding the conserved DB that connects three central helices of D-III of PrV and EBV gH. The present study verified that the conserved DB and several contacting amino acids in D-III modulate cell surface expression and thereby contribute to gH function. In line with this finding, we found that DB C404/C439 and T401 are important for cell-to-cell spread and efficient entry of PrV. This parallel comparison between PrV and EBV gH function brings new insights into how gH structure impacts fusion function during herpesvirus entry.
Importance: The alphaherpesvirus PrV is known for its neuroinvasion, whereas the gammaherpesvirus EBV is associated with cancer of epithelial and B cell origin. Despite low amino acid conservation, PrV gH and EBV gH show strikingly similar structures. Interestingly, both PrV gH and EBV gH contain a structural motif composed of a DB and supporting amino acids which is highly conserved within the Herpesviridae. Our study verified that PrV gH uses a minimal motif with the DB as the core, whereas the DB of EBV gH forms extensive connections through hydrogen bonds to surrounding amino acids, ensuring the cell surface expression of gH/gL. Our study verifies that the comparative analysis of distantly related herpesviruses, such as PrV and EBV, allows the identification of common gH functions. In addition, we provide an understanding of how functional domains can evolve over time, resulting in subtle differences in domain structure and function.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus.Mol Cells. 2016 Apr 30;39(4):286-91. doi: 10.14348/molcells.2016.0066. Epub 2016 Apr 6. Mol Cells. 2016. PMID: 27094060 Free PMC article. Review.
-
The conserved disulfide bond within domain II of Epstein-Barr virus gH has divergent roles in membrane fusion with epithelial cells and B cells.J Virol. 2014 Dec;88(23):13570-9. doi: 10.1128/JVI.02272-14. Epub 2014 Sep 17. J Virol. 2014. PMID: 25231307 Free PMC article.
-
Functional Characterization of Glycoprotein H Chimeras Composed of Conserved Domains of the Pseudorabies Virus and Herpes Simplex Virus 1 Homologs.J Virol. 2015 Oct 21;90(1):421-32. doi: 10.1128/JVI.01985-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26491153 Free PMC article.
-
Structure-based functional analyses of domains II and III of pseudorabies virus glycoprotein H.J Virol. 2015 Jan 15;89(2):1364-76. doi: 10.1128/JVI.02765-14. Epub 2014 Nov 12. J Virol. 2015. PMID: 25392216 Free PMC article.
-
The structural basis of herpesvirus entry.Nat Rev Microbiol. 2021 Feb;19(2):110-121. doi: 10.1038/s41579-020-00448-w. Epub 2020 Oct 21. Nat Rev Microbiol. 2021. PMID: 33087881 Free PMC article. Review.
Cited by
-
Pseudorabies virus infection inhibits autophagy in permissive cells in vitro.Sci Rep. 2017 Jan 6;7:39964. doi: 10.1038/srep39964. Sci Rep. 2017. PMID: 28059118 Free PMC article.
-
EBV gL-gH344-Ferritin Nanoparticle Vaccine Elicits Robust Immune Responses in Mice.Viruses. 2025 May 26;17(6):754. doi: 10.3390/v17060754. Viruses. 2025. PMID: 40573345 Free PMC article.
-
Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus.Mol Cells. 2016 Apr 30;39(4):286-91. doi: 10.14348/molcells.2016.0066. Epub 2016 Apr 6. Mol Cells. 2016. PMID: 27094060 Free PMC article. Review.
References
-
- Longnecker R, Kieff E, Cohen J. 2013. Epstein-Barr virus, 6th ed Lippincott Wilkins and Williams, Philadelphia, PA.
-
- Backovic M, DuBois RM, Cockburn JJ, Sharff AJ, Vaney MC, Granzow H, Klupp BG, Bricogne G, Mettenleiter TC, Rey FA. 2010. Structure of a core fragment of glycoprotein H from pseudorabies virus in complex with antibody. Proc Natl Acad Sci U S A 107:22635–22640. doi:10.1073/pnas.1011507107. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

