Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity
- PMID: 26656754
- PMCID: PMC4677349
- DOI: 10.1038/srep18136
Long-term xeno-free culture of human pluripotent stem cells on hydrogels with optimal elasticity
Abstract
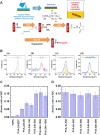
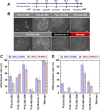
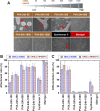
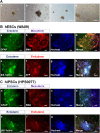
The tentative clinical application of human pluripotent stem cells (hPSCs), such as human embryonic stem cells and human induced pluripotent stem cells, is restricted by the possibility of xenogenic contamination resulting from the use of mouse embryonic fibroblasts (MEFs) as a feeder layer. Therefore, we investigated hPSC cultures on biomaterials with different elasticities that were grafted with different nanosegments. We prepared dishes coated with polyvinylalcohol-co-itaconic acid hydrogels grafted with an oligopeptide derived from vitronectin (KGGPQVTRGDVFTMP) with elasticities ranging from 10.3 to 30.4 kPa storage moduli by controlling the crosslinking time. The hPSCs cultured on the stiffest substrates (30.4 kPa) tended to differentiate after five days of culture, whereas the hPSCs cultured on the optimal elastic substrates (25 kPa) maintained their pluripotency for over 20 passages under xeno-free conditions. These results indicate that cell culture matrices with optimal elasticity can maintain the pluripotency of hPSCs in culture.
Figures




References
-
- Thomson J. A. et al. Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). - PubMed
-
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). - PubMed
-
- Yu J. et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). - PubMed
-
- Rezania A. et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 32, 1121–1133 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

