Exocytotic fusion pores are composed of both lipids and proteins
- PMID: 26656855
- PMCID: PMC4756907
- DOI: 10.1038/nsmb.3141
Exocytotic fusion pores are composed of both lipids and proteins
Abstract
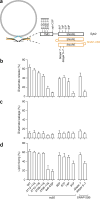
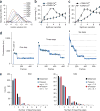
During exocytosis, fusion pores form the first aqueous connection that allows escape of neurotransmitters and hormones from secretory vesicles. Although it is well established that SNARE proteins catalyze fusion, the structure and composition of fusion pores remain unknown. Here, we exploited the rigid framework and defined size of nanodiscs to interrogate the properties of reconstituted fusion pores, using the neurotransmitter glutamate as a content-mixing marker. Efficient Ca(2+)-stimulated bilayer fusion, and glutamate release, occurred with approximately two molecules of mouse synaptobrevin 2 reconstituted into ∼6-nm nanodiscs. The transmembrane domains of SNARE proteins assumed distinct roles in lipid mixing versus content release and were exposed to polar solvent during fusion. Additionally, tryptophan substitutions at specific positions in these transmembrane domains decreased glutamate flux. Together, these findings indicate that the fusion pore is a hybrid structure composed of both lipids and proteins.
Conflict of interest statement
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Figures




Comment in
-
The mystery of the fusion pore.Nat Struct Mol Biol. 2016 Jan;23(1):5-6. doi: 10.1038/nsmb.3157. Nat Struct Mol Biol. 2016. PMID: 26733219 Free PMC article. No abstract available.
References
-
- Rothman JE. The principle of membrane fusion in the cell (Nobel lecture) Angew Chem Int Edn Engl. 2014;53:12676–12694. - PubMed
-
- Bolger AP, et al. Neurohormonal activation and the chronic heart failure syndrome in adults with congenital heart disease. Circulation. 2002;106:92–99. - PubMed
-
- Todde V, Veenhuis M, van der Klei IJ. Autophagy: principles and significance in health and disease. Biochim Biophys Acta. 2009;1792:3–13. - PubMed
-
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

