A CDC42EP4/septin-based perisynaptic glial scaffold facilitates glutamate clearance
- PMID: 26657011
- PMCID: PMC4682051
- DOI: 10.1038/ncomms10090
A CDC42EP4/septin-based perisynaptic glial scaffold facilitates glutamate clearance
Abstract
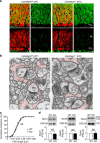
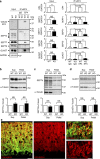
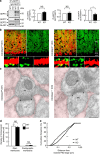
The small GTPase-effector proteins CDC42EP1-5/BORG1-5 interact reciprocally with CDC42 or the septin cytoskeleton. Here we show that, in the cerebellum, CDC42EP4 is exclusively expressed in Bergmann glia and localizes beneath specific membrane domains enwrapping dendritic spines of Purkinje cells. CDC42EP4 forms complexes with septin hetero-oligomers, which interact with a subset of glutamate transporter GLAST/EAAT1. In Cdc42ep4(-/-) mice, GLAST is dissociated from septins and is delocalized away from the parallel fibre-Purkinje cell synapses. The excitatory postsynaptic current exhibits a protracted decay time constant, reduced sensitivity to a competitive inhibitor of the AMPA-type glutamate receptors (γDGG) and excessive baseline inward current in response to a subthreshold dose of a nonselective inhibitor of the glutamate transporters/EAAT1-5 (DL-TBOA). Insufficient glutamate-buffering/clearance capacity in these mice manifests as motor coordination/learning defects, which are aggravated with subthreshold DL-TBOA. We propose that the CDC42EP4/septin-based glial scaffold facilitates perisynaptic localization of GLAST and optimizes the efficiency of glutamate-buffering and clearance.
Figures




References
-
- Tzingounis A. V. & Wadiche J. I. Glutamate transporters: confining runaway excitation by shaping synaptic transmission. Nat. Rev. Neurosci. 8, 935–947 (2007) . - PubMed
-
- Chaudhry F. A. et al. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron 15, 711–720 (1995) . - PubMed
-
- Takayasu Y., Iino M., Takatsuru Y., Tanaka K. & Ozawa S. Functions of glutamate transporters in cerebellar Purkinje cell synapses. Acta Physiol. 197, 1–12 (2009) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

