Metronomic topotecan impedes tumor growth of MYCN-amplified neuroblastoma cells in vitro and in vivo by therapy induced senescence
- PMID: 26657295
- PMCID: PMC4823128
- DOI: 10.18632/oncotarget.6527
Metronomic topotecan impedes tumor growth of MYCN-amplified neuroblastoma cells in vitro and in vivo by therapy induced senescence
Abstract
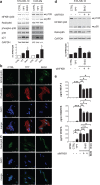
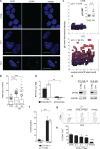
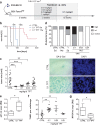
Poor prognosis and frequent relapses are major challenges for patients with high-risk neuroblastoma (NB), especially when tumors show MYCN amplification. High-dose chemotherapy triggers apoptosis, necrosis and senescence, a cellular stress response leading to permanent proliferative arrest and a typical senescence-associated secretome (SASP). SASP components reinforce growth-arrest and act immune-stimulatory, while others are tumor-promoting. We evaluated whether metronomic, i.e. long-term, repetitive low-dose, drug treatment induces senescence in vitro and in vivo. And importantly, by using the secretome as a discriminator for beneficial versus adverse effects of senescence, drugs with a tumor-inhibiting SASP were identified.We demonstrate that metronomic application of chemotherapeutic drugs induces therapy-induced senescence, characterized by cell cycle arrest, p21(WAF/CIP1) up-regulation and DNA double-strand breaks selectively in MYCN-amplified NB. Low-dose topotecan (TPT) was identified as an inducer of a favorable SASP while lacking NFKB1/p50 activation. In contrast, Bromo-deoxy-uridine induced senescent NB-cells secret a tumor-promoting SASP in a NFKB1/p50-dependent manner. Importantly, TPT-treated senescent tumor cells act growth-inhibitory in a dose-dependent manner on non-senescent tumor cells and MYCN expression is significantly reduced in vitro and in vivo. Furthermore, in a mouse xenotransplant-model for MYCN-amplified NB metronomic TPT leads to senescence selectively in tumor cells, complete or partial remission, prolonged survival and a favorable SASP.This new mode-of-action of metronomic TPT treatment, i.e. promoting a tumor-inhibiting type of senescence in MYCN-amplified tumors, is clinically relevant as metronomic regimens are increasingly implemented in therapy protocols of various cancer entities and are considered as a feasible maintenance treatment option with moderate adverse event profiles.
Keywords: MYCN-amplified neuroblastoma; NFKB1; metronomic; senescence-associated-secretory-phenotype; topotecan.
Conflict of interest statement
The authors disclose no potential conflicts of interest.
Figures


Similar articles
-
MicroRNA-149 is associated with clinical outcome in human neuroblastoma and modulates cancer cell proliferation through Rap1 independent of MYCN amplification.Biochimie. 2017 Aug;139:1-8. doi: 10.1016/j.biochi.2017.04.011. Epub 2017 Apr 27. Biochimie. 2017. PMID: 28456710
-
Venetoclax-based Rational Combinations are Effective in Models of MYCN-amplified Neuroblastoma.Mol Cancer Ther. 2021 Aug;20(8):1400-1411. doi: 10.1158/1535-7163.MCT-20-0710. Epub 2021 Jun 4. Mol Cancer Ther. 2021. PMID: 34088831 Free PMC article.
-
A chemical screen identifies the chemotherapeutic drug topotecan as a specific inhibitor of the B-MYB/MYCN axis in neuroblastoma.Oncotarget. 2012 May;3(5):535-45. doi: 10.18632/oncotarget.498. Oncotarget. 2012. PMID: 22619121 Free PMC article.
-
Neuroblastoma: oncogenic mechanisms and therapeutic exploitation of necroptosis.Cell Death Dis. 2015 Dec 3;6(12):e2010. doi: 10.1038/cddis.2015.354. Cell Death Dis. 2015. PMID: 26633716 Free PMC article. Review.
-
Senescence-associated secretory phenotype in lung cancer: remodeling the tumor microenvironment for metastasis and immune suppression.Front Oncol. 2025 May 29;15:1605085. doi: 10.3389/fonc.2025.1605085. eCollection 2025. Front Oncol. 2025. PMID: 40510156 Free PMC article. Review.
Cited by
-
Understanding Cancer's Defense against Topoisomerase-Active Drugs: A Comprehensive Review.Cancers (Basel). 2024 Feb 6;16(4):680. doi: 10.3390/cancers16040680. Cancers (Basel). 2024. PMID: 38398072 Free PMC article. Review.
-
Therapy-Induced Senescence: An "Old" Friend Becomes the Enemy.Cancers (Basel). 2020 Mar 29;12(4):822. doi: 10.3390/cancers12040822. Cancers (Basel). 2020. PMID: 32235364 Free PMC article. Review.
-
Cellular Senescence in the Treatment of Ovarian Cancer.Int J Gynecol Cancer. 2018 Jun;28(5):895-902. doi: 10.1097/IGC.0000000000001257. Int J Gynecol Cancer. 2018. PMID: 29688903 Free PMC article. Review.
-
The Paradoxical Role of Cellular Senescence in Cancer.Front Cell Dev Biol. 2021 Aug 12;9:722205. doi: 10.3389/fcell.2021.722205. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34458273 Free PMC article. Review.
-
Landscape of Bone Marrow Metastasis in Human Neuroblastoma Unraveled by Transcriptomics and Deep Multiplex Imaging.Cancers (Basel). 2021 Aug 26;13(17):4311. doi: 10.3390/cancers13174311. Cancers (Basel). 2021. PMID: 34503120 Free PMC article.
References
-
- Park JR, Bagatell R, London WB, Maris JM, Cohn SL, Mattay KM, Hogarty M, Committee COGN. Children's Oncology Group's 2013 blueprint for research: neuroblastoma. Pediatric blood & cancer. 2013;60:985–993. - PubMed
-
- Cohn SL, Pearson AD, London WB, Monclair T, Ambros PF, Brodeur GM, Faldum A, Hero B, Iehara T, Machin D, Mosseri V, Simon T, Garaventa A, Castel V, Matthay KK. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. Journal of clinical oncology. 2009;27:289–297. - PMC - PubMed
-
- Ambros PF, Ambros IM, Brodeur GM, Haber M, Khan J, Nakagawara A, Schleiermacher G, Speleman F, Spitz R, London WB, Cohn SL, Pearson AD, Maris JM. International consensus for neuroblastoma molecular diagnostics: report from the International Neuroblastoma Risk Group (INRG) Biology Committee. British journal of cancer. 2009;100:1471–1482. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

