Inhibition of EZH2 by chemo- and radiotherapy agents and small molecule inhibitors induces cell death in castration-resistant prostate cancer
- PMID: 26657505
- PMCID: PMC4823118
- DOI: 10.18632/oncotarget.6497
Inhibition of EZH2 by chemo- and radiotherapy agents and small molecule inhibitors induces cell death in castration-resistant prostate cancer
Abstract
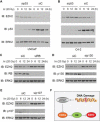
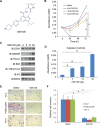
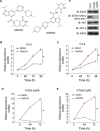
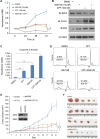
Androgen deprivation therapy is the mainstay of treatment of advanced prostate cancer (PCa). However, a significant portion of patients experience disease relapse and tumors ultimately evolve into castration resistant prostate cancer (CRPC), for which there is no cure in the clinic. The Polycomb protein enhancer of zeste homolog 2 (EZH2) is frequently overexpressed in CRPC. It is unclear whether EZH2 can be a therapeutic target in CRPC. Here, we demonstrated that chemo- and radiotherapy agents such as camptothecin (CPT) and γ irradiation decrease EZH2 expression in various PCa cell lines. We provided evidence that functional p53 and RB proteins are required for CPT- and irradiation-induced downregulation of EZH2 in CRPC cells. We demonstrated that EZH2-specific small molecule inhibitors mitigate CRPC cell growth. We further showed that the EZH2 inhibitor GSK126 inhibits both Polycomb-dependent and -independent functions of EZH2 in PCa cells. Importantly, we found that inhibition of EZH2 by genetic and pharmacological means sensitizes CRPC cells to CPT-induced apoptotic death and growth inhibition in culture and in mice. Our data suggest that concomitant administration of small molecule inhibitors of EZH2 may significantly increase the anti-tumor efficacy of conventional chemo- and radiotherapies in CRPC.
Keywords: EZH2; castration-resistant prostate cancer; chemotherapy; radiation therapy; small molecule inhibitor.
Conflict of interest statement
The authors declare no conflict of interest in this study.
Figures


References
-
- Simon JA, Kingston RE. Mechanisms of polycomb gene silencing: knowns and unknowns. Nat Rev Mol Cell Biol. 2009;10:697–708. - PubMed
-
- Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

