Integrin α4β1 controls G9a activity that regulates epigenetic changes and nuclear properties required for lymphocyte migration
- PMID: 26657637
- PMCID: PMC4838336
- DOI: 10.1093/nar/gkv1348
Integrin α4β1 controls G9a activity that regulates epigenetic changes and nuclear properties required for lymphocyte migration
Abstract
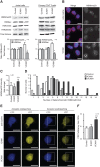
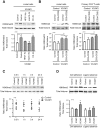
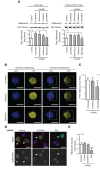
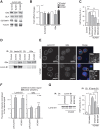
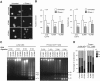
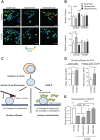
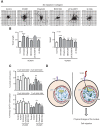
The mechanical properties of the cell nucleus change to allow cells to migrate, but how chromatin modifications contribute to nuclear deformability has not been defined. Here, we demonstrate that a major factor in this process involves epigenetic changes that underpin nuclear structure. We investigated the link between cell adhesion and epigenetic changes in T-cells, and demonstrate that T-cell adhesion to VCAM1 via α4β1 integrin drives histone H3 methylation (H3K9me2/3) through the methyltransferase G9a. In this process, active G9a is recruited to the nuclear envelope and interacts with lamin B1 during T-cell adhesion through α4β1 integrin. G9a activity not only reorganises the chromatin structure in T-cells, but also affects the stiffness and viscoelastic properties of the nucleus. Moreover, we further demonstrated that these epigenetic changes were linked to lymphocyte movement, as depletion or inhibition of G9a blocks T-cell migration in both 2D and 3D environments. Thus, our results identify a novel mechanism in T-cells by which α4β1 integrin signaling drives specific chromatin modifications, which alter the physical properties of the nucleus and thereby enable T-cell migration.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
Similar articles
-
KDM4B histone demethylase and G9a regulate expression of vascular adhesion proteins in cerebral microvessels.Sci Rep. 2017 Mar 22;7:45005. doi: 10.1038/srep45005. Sci Rep. 2017. PMID: 28327608 Free PMC article.
-
Cyclin D1 integrates G9a-mediated histone methylation.Oncogene. 2019 May;38(22):4232-4249. doi: 10.1038/s41388-019-0723-8. Epub 2019 Feb 4. Oncogene. 2019. PMID: 30718920 Free PMC article.
-
G9a/GLP-dependent histone H3K9me2 patterning during human hematopoietic stem cell lineage commitment.Genes Dev. 2012 Nov 15;26(22):2499-511. doi: 10.1101/gad.200329.112. Epub 2012 Oct 26. Genes Dev. 2012. PMID: 23105005 Free PMC article.
-
The role of chromatin repressive marks in cognition and disease: A focus on the repressive complex GLP/G9a.Neurobiol Learn Mem. 2015 Oct;124:88-96. doi: 10.1016/j.nlm.2015.06.013. Epub 2015 Jul 2. Neurobiol Learn Mem. 2015. PMID: 26143996 Review.
-
EHMT2/G9a as an Epigenetic Target in Pediatric and Adult Brain Tumors.Int J Mol Sci. 2021 Oct 19;22(20):11292. doi: 10.3390/ijms222011292. Int J Mol Sci. 2021. PMID: 34681949 Free PMC article. Review.
Cited by
-
Integrins as architects of cell behavior.Mol Biol Cell. 2016 Oct 1;27(19):2885-8. doi: 10.1091/mbc.E15-06-0369. Mol Biol Cell. 2016. PMID: 27687254 Free PMC article. Review.
-
Signal Transduction across the Nuclear Envelope: Role of the LINC Complex in Bidirectional Signaling.Cells. 2019 Feb 4;8(2):124. doi: 10.3390/cells8020124. Cells. 2019. PMID: 30720758 Free PMC article. Review.
-
The Emerging Roles of Heterochromatin in Cell Migration.Front Cell Dev Biol. 2020 May 27;8:394. doi: 10.3389/fcell.2020.00394. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32528959 Free PMC article. Review.
-
Inside the Cell: Integrins as New Governors of Nuclear Alterations?Cancers (Basel). 2017 Jul 6;9(7):82. doi: 10.3390/cancers9070082. Cancers (Basel). 2017. PMID: 28684679 Free PMC article. Review.
-
The Role of Integrin β1D Mislocalization in the Pathophysiology of Calpain 3-Related Limb-Girdle Muscular Dystrophy.Cells. 2025 Mar 17;14(6):446. doi: 10.3390/cells14060446. Cells. 2025. PMID: 40136695 Free PMC article.
References
-
- Lauffenburger D.A., Horwitz A.F. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. - PubMed
-
- Ridley A.J., Schwarts M.A., Burridge K., Firtel R.A., Ginsberg M.H., Borisy G., Parsons J.T., Horwitz A.R. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. - PubMed
-
- Friedl P., Sahai E., Weiss S., Yamada K.M. New dimensions in cell migration. Nat. Rev. Mol. Cell Biol. 2012;13:743–747. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

