Predictive role of hand-foot syndrome in patients receiving first-line capecitabine plus bevacizumab for HER2-negative metastatic breast cancer
- PMID: 26657657
- PMCID: PMC4815806
- DOI: 10.1038/bjc.2015.419
Predictive role of hand-foot syndrome in patients receiving first-line capecitabine plus bevacizumab for HER2-negative metastatic breast cancer
Abstract
Background: Correlations between development of hand-foot syndrome (HFS) and efficacy in patients receiving capecitabine (CAP)-containing therapy are reported in the literature. We explored the relationship between HFS and efficacy in patients receiving CAP plus bevacizumab (BEV) in the TURANDOT randomised phase III trial.
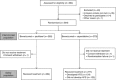
Methods: Patients with HER2-negative locally recurrent/metastatic breast cancer (LR/mBC) who had received no prior chemotherapy for LR/mBC were randomised to BEV plus paclitaxel or BEV-CAP until disease progression or unacceptable toxicity. This analysis included patients randomised to BEV-CAP who received ⩾1 CAP dose. Potential associations between HFS and both overall survival (OS; primary end point) and progression-free survival (PFS; secondary end point) were explored using Cox proportional hazards analyses with HFS as a time-dependent covariate (to avoid overestimating the effect of HFS on efficacy). Landmark analyses were also performed.
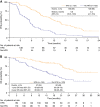
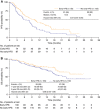
Results: Among 277 patients treated with BEV-CAP, 154 (56%) developed HFS. In multivariate analyses, risk of progression or death was reduced by 44% after the occurrence of HFS; risk of death was reduced by 56%. The magnitude of effect on OS increased with increasing HFS grade. In patients developing HFS within the first 3 months, median PFS from the 3-month landmark was 10.0 months vs 6.2 months in patients without HFS. Two-year OS rates were 63% and 44%, respectively.
Conclusions: This exploratory analysis indicates that HFS occurrence is a strong predictor of prolonged PFS and OS in patients receiving BEV-CAP for LR/mBC. Early appearance of HFS may help motivate patients to continue therapy.
Conflict of interest statement
CZ has received honoraria from and acted as a consultant for Roche; SB has received honoraria from and acted as a consultant for Roche, Pfizer, AbbVie, and Novartis; ZK has acted as a consultant for Roche; DV has received honoraria from Roche and Novartis and has acted as a consultant for Roche and Sanofi Aventis; DM is an employee of a contract research organisation that provides services to F. Hoffmann-La Roche; and TB has received honoraria from Amgen, Bayer, GSK, Eli Lilly, Merck, Novartis, PharmaMar, PrimeOncology, and Roche, and has acted as a consultant for Amgen, Bayer, Novartis, and PharmaMar. The remaining authors declare no conflict of interest.
Figures
References
-
- Andreetta C, Puppin C, Minisini A, Valent F, Pegolo E, Damante G, Di Loreto C, Pizzolitto S, Pandolfi M, Fasola G, Piga A, Puglisi F (2009) Thymidine phosphorylase expression and benefit from capecitabine in patients with advanced breast cancer. Ann Oncol 20: 265–271. - PubMed
-
- Azuma Y, Hata K, Sai K, Udagawa R, Hirakawa A, Tohkin M, Ryushima Y, Makino Y, Yokote N, Morikawa N, Fujiwara Y, Saito Y, Yamamoto H (2012) Significant association between hand-foot syndrome and efficacy of capecitabine in patients with metastatic breast cancer. Biol Pharm Bull 35: 717–724. - PubMed
-
- Bonotto M, Bozza C, Di Loreto C, Osa EO, Poletto E, Puglisi F (2013) Making capecitabine targeted therapy for breast cancer: which is the role of thymidine phosphorylase? Clin Breast Cancer 13: 167–172. - PubMed
-
- Brodowicz T, Lang I, Kahan Z, Greil R, Beslija S, Stemmer SM, Kaufman B, Petruzelka L, Eniu A, Anghel R, Koynov K, Vrbanec D, Pienkowski T, Melichar B, Spanik S, Ahlers S, Messinger D, Inbar MJ, Zielinski C (2014) Selecting first-line bevacizumab-containing therapy for advanced breast cancer: TURANDOT risk factor analyses. Br J Cancer 111: 2051–2057. - PMC - PubMed
-
- Cho JY, Paik YH, Lim HY, Kim YG, Lim HK, Min YW, Gwak GY, Choi MS, Lee JH, Koh KC, Paik SW, Yoo BC (2013) Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int 33: 950–957. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

