Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy
- PMID: 26657860
- PMCID: PMC4701537
- DOI: 10.1172/JCI81217
Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy
Abstract
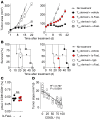
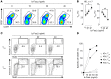
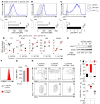
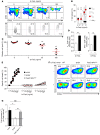
Adoptive cell transfer (ACT) of purified naive, stem cell memory, and central memory T cell subsets results in superior persistence and antitumor immunity compared with ACT of populations containing more-differentiated effector memory and effector T cells. Despite a clear advantage of the less-differentiated populations, the majority of ACT trials utilize unfractionated T cell subsets. Here, we have challenged the notion that the mere presence of less-differentiated T cells in starting populations used to generate therapeutic T cells is sufficient to convey their desirable attributes. Using both mouse and human cells, we identified a T cell-T cell interaction whereby antigen-experienced subsets directly promote the phenotypic, functional, and metabolic differentiation of naive T cells. This process led to the loss of less-differentiated T cell subsets and resulted in impaired cellular persistence and tumor regression in mouse models following ACT. The T memory-induced conversion of naive T cells was mediated by a nonapoptotic Fas signal, resulting in Akt-driven cellular differentiation. Thus, induction of Fas signaling enhanced T cell differentiation and impaired antitumor immunity, while Fas signaling blockade preserved the antitumor efficacy of naive cells within mixed populations. These findings reveal that T cell subsets can synchronize their differentiation state in a process similar to quorum sensing in unicellular organisms and suggest that disruption of this quorum-like behavior among T cells has potential to enhance T cell-based immunotherapies.
Figures




Comment in
-
Selection bias: maintaining less-differentiated T cells for adoptive immunotherapy.J Clin Invest. 2016 Jan;126(1):35-7. doi: 10.1172/JCI85631. Epub 2015 Dec 14. J Clin Invest. 2016. PMID: 26657855 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

