A Novel Three-Dimensional Human Peritubular Microvascular System
- PMID: 26657868
- PMCID: PMC4978055
- DOI: 10.1681/ASN.2015070747
A Novel Three-Dimensional Human Peritubular Microvascular System
Abstract
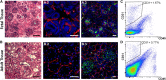
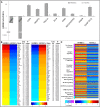
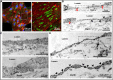
Human kidney peritubular capillaries are particularly susceptible to injury, resulting in dysregulated angiogenesis, capillary rarefaction and regression, and progressive loss of kidney function. However, little is known about the structure and function of human kidney microvasculature. Here, we isolated, purified, and characterized human kidney peritubular microvascular endothelial cells (HKMECs) and reconstituted a three-dimensional human kidney microvasculature in a flow-directed microphysiologic system. By combining epithelial cell depletion and cell culture in media with high concentrations of vascular endothelial growth factor, we obtained HKMECs of high purity in large quantity. Unlike other endothelial cells, isolated HKMECs depended on high vascular endothelial growth factor concentration for survival and growth and exhibited high tubulogenic but low angiogenic potential. Furthermore, HKMECs had a different transcriptional profile. Under flow, HKMECs formed a thin fenestrated endothelium with a functional permeability barrier. In conclusion, this three-dimensional HKMEC-specific microphysiologic system recapitulates human kidney microvascular structure and function and shows phenotypic characteristics different from those of other microvascular endothelial cells.
Keywords: angiogenesis; endothelial cells; fenestrae; kidney; microphysiological system; peritubular microvessels.
Copyright © 2016 by the American Society of Nephrology.
Figures



References
-
- Himmelfarb J, Ikizler TA: Acute kidney injury: Changing lexicography, definitions, and epidemiology. Kidney Int 71: 971–976, 2007 - PubMed
-
- Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 - PubMed
-
- LeBrie SJ: Renal peritubular capillary permeability to macromolecules. Am J Physiol 213: 1225–1232, 1967 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

