The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans
- PMID: 26657939
- PMCID: PMC4675080
- DOI: 10.1038/srep18015
The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans
Abstract
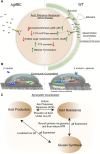
Streptococcus mutans is considered the principal cariogenic bacterium for dental caries. Despite the recognition of their importance for cariogenesis, the possible coordination among S. mutans' main virulence factors, including glucan production, acidogenicity and aciduricity, has been less well studied. In the present study, using S. mutans strains with surface-displayed pH-sensitive pHluorin, we revealed sucrose availability- and Gtf functionality-dependent proton accumulation on S. mutans surface. Consistent with this, using a pH-sensitive dye, we demonstrated that both in vivo cell-produced and in vitro enzymatically synthesized insoluble glucans displayed proton-concentrating ability. Global transcriptomics revealed proton accumulation triggers the up-regulation of genes encoding functions involved in acid tolerance response in a glucan-dependent manner. Our data suggested that this proton enrichment around S. mutans could pre-condition the bacterium for acid-stress. Consistent with this hypothesis, we found S. mutans strains defective in glucan production were more acid sensitive. Our study revealed for the first time that insoluble glucans is likely an essential factor linking acidogenicity with aciduricity. The coordination of these key virulence factors could provide new insights on how S. mutans may have become a major cariogenic pathogen.
Conflict of interest statement
The authors declare that W.S. is a part time Chief Science Officer of C3 Jian Inc., which has licensed technologies from UC Regents that could be indirectly related to this research project.
Figures


Similar articles
-
Regulation of water-soluble glucan synthesis by the Streptococcus mutans dexA gene effects biofilm aggregation and cariogenic pathogenicity.Mol Oral Microbiol. 2019 Apr;34(2):51-63. doi: 10.1111/omi.12253. Epub 2019 Feb 14. Mol Oral Microbiol. 2019. PMID: 30659765
-
Glycosyltransferase-Mediated Biofilm Matrix Dynamics and Virulence of Streptococcus mutans.Appl Environ Microbiol. 2019 Feb 20;85(5):e02247-18. doi: 10.1128/AEM.02247-18. Print 2019 Mar 1. Appl Environ Microbiol. 2019. PMID: 30578260 Free PMC article.
-
Betulin inhibits cariogenic properties of Streptococcus mutans by targeting vicRK and gtf genes.Antonie Van Leeuwenhoek. 2017 Jan;110(1):153-165. doi: 10.1007/s10482-016-0785-3. Epub 2016 Oct 18. Antonie Van Leeuwenhoek. 2017. PMID: 27757704
-
Genomic and phenotypic diversity of Streptococcus mutans.J Oral Biosci. 2019 Mar;61(1):22-31. doi: 10.1016/j.job.2018.11.001. Epub 2018 Nov 15. J Oral Biosci. 2019. PMID: 30929798 Review.
-
[Development of transcriptional regulators of Streptococcus mutans in cariogenic virulence].Hua Xi Kou Qiang Yi Xue Za Zhi. 2016 Dec 1;34(6):643-646. doi: 10.7518/hxkq.2016.06.018. Hua Xi Kou Qiang Yi Xue Za Zhi. 2016. PMID: 28318169 Free PMC article. Review. Chinese.
Cited by
-
Inactivation of the spxA1 or spxA2 gene of Streptococcus mutans decreases virulence in the rat caries model.Mol Oral Microbiol. 2017 Apr;32(2):142-153. doi: 10.1111/omi.12160. Epub 2016 May 16. Mol Oral Microbiol. 2017. PMID: 27037617 Free PMC article.
-
Inactivation of Streptococcus mutans genes lytST and dltAD impairs its pathogenicity in vivo.J Oral Microbiol. 2019 May 9;11(1):1607505. doi: 10.1080/20002297.2019.1607505. eCollection 2019. J Oral Microbiol. 2019. PMID: 31143407 Free PMC article.
-
pH-Sensitive Compounds for Selective Inhibition of Acid-Producing Bacteria.ACS Appl Mater Interfaces. 2018 Mar 14;10(10):8566-8573. doi: 10.1021/acsami.8b01089. Epub 2018 Feb 28. ACS Appl Mater Interfaces. 2018. PMID: 29436821 Free PMC article.
-
Exopolysaccharides metabolism and cariogenesis of Streptococcus mutans biofilm regulated by antisense vicK RNA.J Oral Microbiol. 2023 Apr 28;15(1):2204250. doi: 10.1080/20002297.2023.2204250. eCollection 2023. J Oral Microbiol. 2023. PMID: 37138664 Free PMC article.
-
[Role of SMU.2055 gene in cariogenic capacity of Streptococcus mutans].Nan Fang Yi Ke Da Xue Xue Bao. 2017 Jun 20;37(6):786-791. doi: 10.3969/j.issn.1673-4254.2017.06.12. Nan Fang Yi Ke Da Xue Xue Bao. 2017. PMID: 28669953 Free PMC article. Chinese.
References
-
- Selwitz R. H., Ismail A. I. & Pitts N. B. Dental caries. Lancet 369, 51–59 (2007). - PubMed
-
- Marsh P. D. Microbial ecology of dental plaque and its significance in health and disease. Adv Dent Res 8, 263–271 (1994). - PubMed
-
- Colby S. M. & Russell R. R. Sugar metabolism by mutans streptococci. Soc Appl Bacteriol Symp Ser. 26, 80S–88S (1997). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

