Vibrio Iron Transport: Evolutionary Adaptation to Life in Multiple Environments
- PMID: 26658001
- PMCID: PMC4711184
- DOI: 10.1128/MMBR.00046-15
Vibrio Iron Transport: Evolutionary Adaptation to Life in Multiple Environments
Abstract
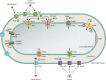
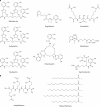
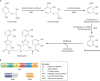
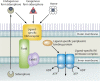
Iron is an essential element for Vibrio spp., but the acquisition of iron is complicated by its tendency to form insoluble ferric complexes in nature and its association with high-affinity iron-binding proteins in the host. Vibrios occupy a variety of different niches, and each of these niches presents particular challenges for acquiring sufficient iron. Vibrio species have evolved a wide array of iron transport systems that allow the bacteria to compete for this essential element in each of its habitats. These systems include the secretion and uptake of high-affinity iron-binding compounds (siderophores) as well as transport systems for iron bound to host complexes. Transporters for ferric and ferrous iron not complexed to siderophores are also common to Vibrio species. Some of the genes encoding these systems show evidence of horizontal transmission, and the ability to acquire and incorporate additional iron transport systems may have allowed Vibrio species to more rapidly adapt to new environmental niches. While too little iron prevents growth of the bacteria, too much can be lethal. The appropriate balance is maintained in vibrios through complex regulatory networks involving transcriptional repressors and activators and small RNAs (sRNAs) that act posttranscriptionally. Examination of the number and variety of iron transport systems found in Vibrio spp. offers insights into how this group of bacteria has adapted to such a wide range of habitats.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Similar articles
-
Catechol Siderophore Transport by Vibrio cholerae.J Bacteriol. 2015 Sep;197(17):2840-9. doi: 10.1128/JB.00417-15. Epub 2015 Jun 22. J Bacteriol. 2015. PMID: 26100039 Free PMC article.
-
Identification of genes, desR and desA, required for utilization of desferrioxamine B as a xenosiderophore in Vibrio furnissii.Biol Pharm Bull. 2011;34(4):570-4. doi: 10.1248/bpb.34.570. Biol Pharm Bull. 2011. PMID: 21467648
-
Characterization of the angR gene of Vibrio anguillarum: essential role in virulence.Infect Immun. 1999 Dec;67(12):6496-509. doi: 10.1128/IAI.67.12.6496-6509.1999. Infect Immun. 1999. PMID: 10569768 Free PMC article.
-
Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria.Microbiol Mol Biol Rev. 1997 Sep;61(3):319-36. doi: 10.1128/mmbr.61.3.319-336.1997. Microbiol Mol Biol Rev. 1997. PMID: 9293185 Free PMC article. Review.
-
Characterization of ferric-anguibactin transport in Vibrio anguillarum.Biometals. 2007 Jun;20(3-4):393-403. doi: 10.1007/s10534-007-9084-9. Epub 2007 Feb 8. Biometals. 2007. PMID: 17287889 Review.
Cited by
-
Siderophore piracy enhances Vibrio cholerae environmental survival and pathogenesis.Microbiology (Reading). 2020 Nov;166(11):1038-1046. doi: 10.1099/mic.0.000975. Epub 2020 Oct 5. Microbiology (Reading). 2020. PMID: 33074088 Free PMC article.
-
In vivo test of Vibrio alginolyticus and Vibrio harveyi infection in the humpback grouper (Cromileptes altivelis) from East Java Indonesia.Vet World. 2022 May;15(5):1269-1282. doi: 10.14202/vetworld.2022.1269-1282. Epub 2022 May 23. Vet World. 2022. PMID: 35765493 Free PMC article.
-
Axial Heme Coordination by the Tyr-His Motif in the Extracellular Hemophore HasAp Is Critical for the Release of Heme to the HasR Receptor of Pseudomonas aeruginosa.Biochemistry. 2021 Aug 24;60(33):2549-2559. doi: 10.1021/acs.biochem.1c00389. Epub 2021 Jul 29. Biochemistry. 2021. PMID: 34324310 Free PMC article.
-
Increasing the copper sensitivity of microorganisms by restricting iron supply, a strategy for bio-management practices.Microb Biotechnol. 2020 Sep;13(5):1530-1545. doi: 10.1111/1751-7915.13590. Epub 2020 Jun 19. Microb Biotechnol. 2020. PMID: 32558275 Free PMC article.
-
Insights into the Vibrio Genus: A One Health Perspective from Host Adaptability and Antibiotic Resistance to In Silico Identification of Drug Targets.Antibiotics (Basel). 2022 Oct 12;11(10):1399. doi: 10.3390/antibiotics11101399. Antibiotics (Basel). 2022. PMID: 36290057 Free PMC article.
References
-
- Farmer JJI. 2006. The family Vibrionaceae, p 495–507. In Dworkin M, Falkow S, Rosenberg E, Schleifer K-H, Stackebrandt E (ed), The prokaryotes. Springer, New York, NY.
-
- Crosa JH, Payne SM, Mey AR. 2004. Iron transport in bacteria. ASM Press, Washington, DC.
-
- Ju C-H, Yeung PSM, Oesterling J, Seigerman DA, Boor KJ. 2006. Vibrio parahaemolyticus growth under low-iron conditions and survival under high-magnesium conditions. J Food Prot 69:1040–1045. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

