MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints
- PMID: 26658052
- PMCID: PMC4827047
- DOI: 10.1093/neuonc/nov292
MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints
Abstract
Background: Antibody therapeutic targeting of the immune checkpoints cytotoxic T-lymphocyte-associated molecule 4 (CTLA-4) and programmed cell death 1 (PD-1) has demonstrated marked tumor regression in clinical trials. MicroRNAs (miRNAs) can modulate multiple gene transcripts including possibly more than one immune checkpoint and could be exploited as immune therapeutics.
Methods: Using online miRNA targeting prediction algorithms, we searched for miRNAs that were predicted to target both PD-1 and CTLA-4. MiR-138 emerged as a leading candidate. The effects of miR-138 on CTLA-4 and PD-1 expression and function in T cells were determined and the therapeutic effect of intravenous administration of miR-138 was investigated in both immune-competent and -incompetent murine models of GL261 glioma.
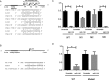
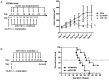
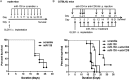
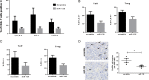
Results: Target binding algorithms predicted that miR-138 could bind the 3' untranslated regions of CTLA-4 and PD-1, which was confirmed with luciferase expression assays. Transfection of human CD4+ T cells with miR-138 suppressed expression of CTLA-4, PD-1, and Forkhead box protein 3 (FoxP3) in transfected human CD4+ T cells. In vivo miR-138 treatment of GL261 gliomas in immune-competent mice demonstrated marked tumor regression, a 43% increase in median survival time (P = .011), and an associated decrease in intratumoral FoxP3+ regulatory T cells, CTLA-4, and PD-1 expression. This treatment effect was lost in nude immune-incompetent mice and with depletion of CD4+ or CD8+ T cells, and miR-138 had no suppressive effect on glioma cells when treated directly at physiological in vivo doses.
Conclusions: MiR-138 exerts anti-glioma efficacy by targeting immune checkpoints which may have rapid translational potential as a novel immunotherapeutic agent.
Keywords: CTLA-4; PD-1; glioblastoma; miR-138; microRNAs.
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
MicroRNAs provide a novel pathway toward combinatorial immune checkpoint blockade.Neuro Oncol. 2016 May;18(5):601-2. doi: 10.1093/neuonc/now003. Epub 2016 Mar 14. Neuro Oncol. 2016. PMID: 26980424 Free PMC article. No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. - PubMed
-
- Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. - PubMed
-
- Chan JA, Krichevsky AM, Kosik KS. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005;65(14):6029–6033. - PubMed
-
- Ciafre SA, Galardi S, Mangiola A et al. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334(4):1351–1358. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

