AVERT2 (a very early rehabilitation trial, a very effective reproductive trigger): retrospective observational analysis of the number of babies born to trial staff
- PMID: 26658193
- PMCID: PMC4676118
- DOI: 10.1136/bmj.h6432
AVERT2 (a very early rehabilitation trial, a very effective reproductive trigger): retrospective observational analysis of the number of babies born to trial staff
Abstract
Objective: To report the number of participants needed to recruit per baby born to trial staff during AVERT, a large international trial on acute stroke, and to describe trial management consequences.
Design: Retrospective observational analysis.
Setting: 56 acute stroke hospitals in eight countries.
Participants: 1074 trial physiotherapists, nurses, and other clinicians.
Outcome measures: Number of babies born during trial recruitment per trial participant recruited.
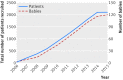
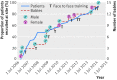
Results: With 198 site recruitment years and 2104 patients recruited during AVERT, 120 babies were born to trial staff. Births led to an estimated 10% loss in time to achieve recruitment. Parental leave was linked to six trial site closures. The number of participants needed to recruit per baby born was 17.5 (95% confidence interval 14.7 to 21.0); additional trial costs associated with each birth were estimated at 5736 Australian dollars on average.
Conclusion: The staff absences registered in AVERT owing to parental leave led to delayed trial recruitment and increased costs, and should be considered by trial investigators when planning research and estimating budgets. However, the celebration of new life became a highlight of the annual AVERT collaborators' meetings and helped maintain a cohesive collaborative group.
Trial registration: Australian New Zealand Clinical Trials Registry no 12606000185561.
Disclaimer: Participation in a rehabilitation trial does not guarantee successful reproductive activity.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at
Figures
References
-
- Bernhardt J, Churilov L, Dewey Het al.AVERT Collaborators. Statistical analysis plan (SAP) for A Very Early Rehabilitation Trial (AVERT): an international trial to determine the efficacy and safety of commencing out of bed standing and walking training (very early mobilization) within 24 h of stroke onset vs. usual stroke unit care. Int J Stroke 2015;10: 23-4. 10.1111/ijs.12423 25491547 - DOI - PubMed
-
- Australian Institute of Health and Welfare. Nursing and Midwifery Workforce 2012. Canberra, Australia: 2013 Contract No: HWL 52.
-
- Australian Institute of Health and Welfare. Allied Health Workforce 2012. Canberra: 2013 Contract No: HWL 51.
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical