Ethanol Inhibits High-Affinity Immunoglobulin E Receptor (FcεRI) Signaling in Mast Cells by Suppressing the Function of FcεRI-Cholesterol Signalosome
- PMID: 26658290
- PMCID: PMC4686000
- DOI: 10.1371/journal.pone.0144596
Ethanol Inhibits High-Affinity Immunoglobulin E Receptor (FcεRI) Signaling in Mast Cells by Suppressing the Function of FcεRI-Cholesterol Signalosome
Abstract
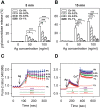
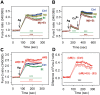
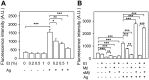
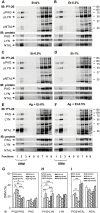
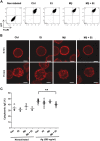
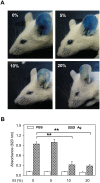
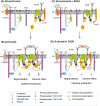
Ethanol has multiple effects on biochemical events in a variety of cell types, including the high-affinity immunoglobulin E receptor (FcεRI) signaling in antigen-activated mast cells. However, the underlying molecular mechanism remains unknown. To get better understanding of the effect of ethanol on FcεRI-mediated signaling we examined the effect of short-term treatment with non-toxic concentrations of ethanol on FcεRI signaling events in mouse bone marrow-derived mast cells. We found that 15 min exposure to ethanol inhibited antigen-induced degranulation, calcium mobilization, expression of proinflammatory cytokine genes (tumor necrosis factor-α, interleukin-6, and interleukin-13), and formation of reactive oxygen species in a dose-dependent manner. Removal of cellular cholesterol with methyl-β-cyclodextrin had a similar effect and potentiated some of the inhibitory effects of ethanol. In contrast, exposure of the cells to cholesterol-saturated methyl-β-cyclodextrin abolished in part the inhibitory effect of ethanol on calcium response and production of reactive oxygen species, supporting lipid-centric theories of ethanol action on the earliest stages of mast cell signaling. Further studies showed that exposure to ethanol and/or removal of cholesterol inhibited early FcεRI activation events, including tyrosine phosphorylation of the FcεRI β and γ subunits, SYK kinases, LAT adaptor protein, phospholipase Cγ, STAT5, and AKT and internalization of aggregated FcεRI. Interestingly, ethanol alone, and particularly in combination with methyl-β-cyclodextrin, enhanced phosphorylation of negative regulatory tyrosine 507 of LYN kinase. Finally, we found that ethanol reduced passive cutaneous anaphylactic reaction in mice, suggesting that ethanol also inhibits FcεRI signaling under in vivo conditions. The combined data indicate that ethanol interferes with early antigen-induced signaling events in mast cells by suppressing the function of FcεRI-cholesterol signalosomes at the plasma membrane.
Conflict of interest statement
Figures




References
-
- Meyer HH. Zur theorie der alkoholnarkose. I. Mitt. welche eigenschaft der anästhetika bedingt ihre narkotische wirkung? Arch Exp Pathol Pharmakol. 1899; 42: 109–108.
-
- Meyer KH. Contribution to the theory of narcosis. Trans Faraday Soc. 1937; 33: 1062–1068.
-
- Chin JH, Goldstein DB. Effects of low concentrations of ethanol on the fluidity of spin-labeled erythrocyte and brain membranes. Mol Pharmacol. 1977; 13: 435–441. - PubMed
-
- Chen SY, Yang B, Jacobson K, Sulik KK. The membrane disordering effect of ethanol on neural crest cells in vitro and the protective role of GM1 ganglioside. Alcohol. 1996; 13: 589–595. S0741832996000730. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

