RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders
- PMID: 26658372
- PMCID: PMC4677355
- DOI: 10.1038/srep18193
RNA-mediated paternal heredity of diet-induced obesity and metabolic disorders
Abstract
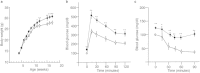
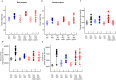
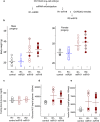
The paternal heredity of obesity and diabetes induced by a high-fat and/or high-sugar diet (Western-like diet) has been demonstrated through epidemiological analysis of human cohorts and experimental analysis, but the nature of the hereditary vector inducing this newly acquired phenotype is not yet well defined. Here, we show that microinjection of either testis or sperm RNA of male mice fed a Western-like diet into naive one-cell embryos leads to the establishment of the Western-like diet-induced metabolic phenotype in the resulting progenies, whereas RNAs prepared from healthy controls did not. Among multiple sequence differences between the testis transcriptomes of the sick and healthy fathers, we noted that several microRNAs had increased expression, which was of interest because this class of noncoding RNA is known to be involved in epigenetic control of gene expression. When microinjected into naive one-cell embryos, one of these small RNA, i.e., the microRNA miR19b, induced metabolic alterations that are similar to the diet-induced phenotype. Furthermore, this pathological phenotype was inherited by the offspring after crosses with healthy partners. Our results indicate that acquired food-induced trait inheritance might be enacted by RNA signalling.
Figures

References
-
- Chen L., Magliano D. J. & Zimmet P. Z. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol 8, 228–236 (2012). - PubMed
-
- Painter R. C. et al. Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG : an international journal of obstetrics and gynaecology 115, 1243–1249 (2008). - PubMed
-
- Veenendaal M. V. et al. Transgenerational effects of prenatal exposure to the 1944-45 Dutch famine. BJOG : an international journal of obstetrics and gynaecology 120, 548–553 (2013). - PubMed
-
- Kaati G., Bygren L. O., Pembrey M. & Sjostrom M. Transgenerational response to nutrition, early life circumstances and longevity. Eur J Hum Genet 15, 784–790 (2007). - PubMed
-
- Ng S. F. et al. Chronic high-fat diet in fathers programs beta-cell dysfunction in female rat offspring. Nature 467, 963–966 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

