Subconjunctivally Implanted Hydrogels for Sustained Insulin Release to Reduce Retinal Cell Apoptosis in Diabetic Rats
- PMID: 26658505
- PMCID: PMC4682486
- DOI: 10.1167/iovs.15-16998
Subconjunctivally Implanted Hydrogels for Sustained Insulin Release to Reduce Retinal Cell Apoptosis in Diabetic Rats
Abstract
Purpose: Diabetic retinopathy (DR) is a leading cause of blindness in diabetic patients that involves early-onset retinal cell loss. Here, we report our recent work using subconjunctivally implantable hydrogels for sustained insulin release to the retina to prevent retinal degeneration.
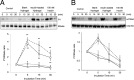
Methods: The hydrogels are synthesized by UV photopolymerization of N-isopropylacrylamide and a dextran macromer containing oligolactate-(2-hydroxyetheyl methacrylate) units. Insulin was loaded into the hydrogels during the synthesis. The ex vivo bioactivity of insulin released from the hydrogels was tested on fresh rat retinas using immunoprecipitation and immunoblotting to measure insulin receptor tyrosine and Akt phosphorylation. The biosafety and the effect on the blood glucose of the hydrogels were evaluated in rats 2 months after subconjunctival implantation. The release of insulin from the hydrogels was studied both in vitro in PBS (pH 7.4), and in vivo using confocal microscopy and RIA kit. The in vivo bioactivity of the released insulin was investigated in diabetic rats using DNA fragmentation method.
Results: The hydrogels could load insulin with approximately 98% encapsulation efficiency and continuously release FITC-insulin in PBS (pH = 7.4) at 37°C for at least 5 months depending on their composition. Insulin lispro released from the hydrogels was biologically active by increasing insulin receptor tyrosine and Akt serine phosphorylation of ex vivo retinas. In vivo studies showed normal retinal histology 2 months post subconjunctival implantation. Insulin released from subconjunctivally implanted hydrogels could be detected in the retina by using confocal microscopy and RIA kit for 1 week. The implanted hydrogels with insulin lispro did not change the blood glucose level of normal and diabetic rats, but significantly reduced the DNA fragmentation of diabetic retinas for 1 week.
Conclusions: The developed hydrogels have great potential to sustain release of insulin to the retina via subconjunctival implantation to minimize DR without the risk of hypoglycemia.
Figures






References
-
- National Diabetes Statistics Report. 2014. Available at: http://www.cdc.gov/diabetes/data/statistics/2014StatisticsReport.html.Accessed February 7, 2015.
-
- Ip MS,, Domalpally A,, Hopkins JJ,, Wong P,, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012; 130: 1145–1152. - PubMed
-
- Gardner TW,, Antonetti DA,, Barber AJ,, LaNoue KF,, Levison SW. Diabetic retinopathy: more than meets the eye. Surv Ophthalmol. 2002; 47: S253–S262. - PubMed
-
- Antonetti DA,, Barber AJ,, Bronson SK,, et al. Diabetic retinopathy—seeing beyond glucose-induced microvascular disease. Diabetes. 2006; 55: 2401–2411. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

