LIM Kinase, a Newly Identified Regulator of Presynaptic Remodeling by Rod Photoreceptors After Injury
- PMID: 26658506
- PMCID: PMC4682489
- DOI: 10.1167/iovs.15-17278
LIM Kinase, a Newly Identified Regulator of Presynaptic Remodeling by Rod Photoreceptors After Injury
Abstract
Purpose: Rod photoreceptors retract their axon terminals and develop neuritic sprouts in response to retinal detachment and reattachment, respectively. This study examines the role of LIM kinase (LIMK), a component of RhoA and Rac pathways, in the presynaptic structural remodeling of rod photoreceptors.
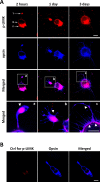
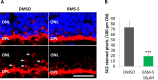
Methods: Phosphorylated LIMK (p-LIMK), the active form of LIMK, was examined in salamander retina with Western blot and confocal microscopy. Axon length within the first 7 hours and process growth after 3 days of culture were assessed in isolated rod photoreceptors treated with inhibitors of upstream regulators ROCK and p21-activated kinase (Pak) (Y27632 and IPA-3) and a direct LIMK inhibitor (BMS-5). Porcine retinal explants were also treated with BMS-5 and analyzed 24 hours after detachment. Because Ca2+ influx contributes to axonal retraction, L-type channels were blocked in some experiments with nicardipine.
Results: Phosphorylated LIMK is present in rod terminals during retraction and in newly formed processes. Axonal retraction over 7 hours was significantly reduced by inhibition of LIMK or its regulators, ROCK and Pak. Process growth was reduced by LIMK or Pak inhibition especially at the basal (axon-bearing) region of the rod cells. Combining Ca2+ channel and LIMK inhibition had no additional effect on retraction but did further inhibit sprouting after 3 days. In detached porcine retina, LIMK inhibition reduced rod axonal retraction and improved retinal morphology.
Conclusions: Thus structural remodeling, in the form of either axonal retraction or neuritic growth, requires LIMK activity. LIM kinase inhibition may have therapeutic potential for reducing pathologic rod terminal plasticity after retinal injury.
Figures







Similar articles
-
Sema3A Reduces Sprouting of Adult Rod Photoreceptors In Vitro.Invest Ophthalmol Vis Sci. 2017 Aug 1;58(10):4318–4331. doi: 10.1167/iovs.16-21075. Invest Ophthalmol Vis Sci. 2017. PMID: 28806446 Free PMC article.
-
Actin Dynamics, Regulated by RhoA-LIMK-Cofilin Signaling, Mediates Rod Photoreceptor Axonal Retraction After Retinal Injury.Invest Ophthalmol Vis Sci. 2019 May 1;60(6):2274-2285. doi: 10.1167/iovs.18-26077. Invest Ophthalmol Vis Sci. 2019. PMID: 31112612 Free PMC article.
-
RhoA and its role in synaptic structural plasticity of isolated salamander photoreceptors.Invest Ophthalmol Vis Sci. 2008 Sep;49(9):4177-87. doi: 10.1167/iovs.07-1580. Epub 2008 May 23. Invest Ophthalmol Vis Sci. 2008. PMID: 18503000
-
Lim kinase, a bi-functional effector in injury-induced structural plasticity of synapses.Neural Regen Res. 2016 Jul;11(7):1029-32. doi: 10.4103/1673-5374.187018. Neural Regen Res. 2016. PMID: 27630670 Free PMC article. Review.
-
Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment.Prog Retin Eye Res. 2005 May;24(3):395-431. doi: 10.1016/j.preteyeres.2004.10.004. Epub 2005 Jan 13. Prog Retin Eye Res. 2005. PMID: 15708835 Review.
Cited by
-
ROCK inhibition reduces morphological and functional damage to rod synapses after retinal injury.Sci Rep. 2021 Jan 12;11(1):692. doi: 10.1038/s41598-020-80267-4. Sci Rep. 2021. PMID: 33436892 Free PMC article.
-
Sema3A Reduces Sprouting of Adult Rod Photoreceptors In Vitro.Invest Ophthalmol Vis Sci. 2017 Aug 1;58(10):4318–4331. doi: 10.1167/iovs.16-21075. Invest Ophthalmol Vis Sci. 2017. PMID: 28806446 Free PMC article.
-
Actin Dynamics, Regulated by RhoA-LIMK-Cofilin Signaling, Mediates Rod Photoreceptor Axonal Retraction After Retinal Injury.Invest Ophthalmol Vis Sci. 2019 May 1;60(6):2274-2285. doi: 10.1167/iovs.18-26077. Invest Ophthalmol Vis Sci. 2019. PMID: 31112612 Free PMC article.
-
Coming of Age for the Photoreceptor Synapse.Invest Ophthalmol Vis Sci. 2021 Sep 2;62(12):24. doi: 10.1167/iovs.62.12.24. Invest Ophthalmol Vis Sci. 2021. PMID: 34550300 Free PMC article.
-
Novel Therapeutics in Glaucoma Management.Curr Neuropharmacol. 2018;16(7):978-992. doi: 10.2174/1570159X15666170915142727. Curr Neuropharmacol. 2018. PMID: 28925883 Free PMC article. Review.
References
-
- Erickson PA,, Fisher SK,, Anderson DH,, Stern WH,, Borgula GA. Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci. 1983; 24: 927–942. - PubMed
-
- Lewis GP,, Linberg KA,, Fisher SK. Neurite outgrowth from bipolar and horizontal cells after experimental retinal detachment. Invest Ophthalmol Vis Sci. 1998; 39: 424–434. - PubMed
-
- Milam AH,, Li ZY,, Cideciyan AV,, Jacobson SG. Clinicopathologic effects of the Q64ter rhodopsin mutation in retinitis pigmentosa. Invest Ophthalmol Vis Sci. 1996; 37: 753–765. - PubMed
-
- Lewis GP,, Charteris DG,, Sethi CS,, Fisher SK. Animal models of retinal detachment and reattachment: identifying cellular events that may affect visual recovery. Eye (Lond). 2002; 16: 375–387. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

