Variant fatty acid-like molecules Conjugation, novel approaches for extending the stability of therapeutic peptides
- PMID: 26658631
- PMCID: PMC4676015
- DOI: 10.1038/srep18039
Variant fatty acid-like molecules Conjugation, novel approaches for extending the stability of therapeutic peptides
Abstract
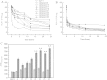
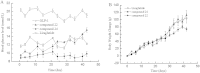
The multiple physiological properties of glucagon-like peptide-1 (GLP-1) make it a promising drug candidate for the treatment of type 2 diabetes. However, the in vivo half-life of GLP-1 is short due to rapid degradation by dipeptidyl peptidase-IV (DPP-IV) and renal clearance. The poor stability of GLP-1 has significantly limited its clinical utility; however, many studies are focused on extending its stability. Fatty acid conjugation is a traditional approach for extending the stability of therapeutic peptides because of the high binding affinity of human serum albumin for fatty acids. However, the conjugate requires a complex synthetic approach, usually involving Lys and occasionally involving a linker. In the current study, we conjugated the GLP-1 molecule with fatty acid derivatives to simplify the synthesis steps. Human serum albumin binding assays indicated that the retained carboxyl groups of the fatty acids helped maintain a tight affinity to HSA. The conjugation of fatty acid-like molecules improved the stability and increased the binding affinity of GLP-1 to HSA. The use of fatty acid-like molecules as conjugating components allowed variant conjugation positions and freed carboxyl groups for other potential uses. This may be a novel, long-acting strategy for the development of therapeutic peptides.
Figures



Similar articles
-
A novel GLP-1 analog, BPI3006, with potent DPP IV resistance and good glucoregulatory effect.Biochem Biophys Res Commun. 2010 Oct 1;400(4):563-8. doi: 10.1016/j.bbrc.2010.08.103. Epub 2010 Sep 6. Biochem Biophys Res Commun. 2010. PMID: 20804731
-
Novel application of hydrophobin in medical science: a drug carrier for improving serum stability.Sci Rep. 2016 May 23;6:26461. doi: 10.1038/srep26461. Sci Rep. 2016. PMID: 27212208 Free PMC article.
-
Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV.Endocrinology. 1995 Aug;136(8):3585-96. doi: 10.1210/endo.136.8.7628397. Endocrinology. 1995. PMID: 7628397
-
Glucagon-like peptide 1 and inhibitors of dipeptidyl peptidase IV in the treatment of type 2 diabetes mellitus.Curr Opin Pharmacol. 2004 Dec;4(6):589-96. doi: 10.1016/j.coph.2004.08.005. Curr Opin Pharmacol. 2004. PMID: 15525549 Review.
-
Beyond glucose lowering: glucagon-like peptide-1 receptor agonists, body weight and the cardiovascular system.Diabetes Metab. 2011 Dec;37(6):477-88. doi: 10.1016/j.diabet.2011.07.001. Epub 2011 Aug 25. Diabetes Metab. 2011. PMID: 21871831 Review.
Cited by
-
Lipopeptide-mediated Cas9 RNP delivery: A promising broad therapeutic strategy for safely removing deep-intronic variants in ABCA4.Mol Ther Nucleic Acids. 2024 Sep 26;35(4):102345. doi: 10.1016/j.omtn.2024.102345. eCollection 2024 Dec 10. Mol Ther Nucleic Acids. 2024. PMID: 39494150 Free PMC article.
-
Glycation of human serum albumin impairs binding to the glucagon-like peptide-1 analogue liraglutide.J Biol Chem. 2018 Mar 30;293(13):4778-4791. doi: 10.1074/jbc.M117.815274. Epub 2018 Feb 2. J Biol Chem. 2018. PMID: 29414771 Free PMC article.
-
Factors affecting the physical stability (aggregation) of peptide therapeutics.Interface Focus. 2017 Dec 6;7(6):20170030. doi: 10.1098/rsfs.2017.0030. Epub 2017 Oct 20. Interface Focus. 2017. PMID: 29147559 Free PMC article. Review.
-
Understanding peptide hormones: from precursor proteins to bioactive molecules.Trends Biochem Sci. 2025 Jun;50(6):481-494. doi: 10.1016/j.tibs.2025.03.014. Epub 2025 Apr 14. Trends Biochem Sci. 2025. PMID: 40234176 Review.
-
Leveraging RAS-mSIN1 interaction to selectively inhibit mTORC2 employing competitive RAS binding peptide: implications in breast cancer metastasis.Oncogene. 2025 Aug 23. doi: 10.1038/s41388-025-03516-8. Online ahead of print. Oncogene. 2025. PMID: 40847128
References
-
- Curry S., Brick P. & Franks N. P. Fatty acid binding to human serum albumin: new insights from crystallographic studies. Biochim Biophys Acta 1441, 131–140, doi: S1388-1981(99)00148-1 (1999). - PubMed
-
- Bhattacharya A. A., Grune T. & Curry S. Crystallographic analysis reveals common modes of binding of medium and long-chain fatty acids to human serum albumin. J Mol Biol 303, 721–732 (2000). - PubMed
-
- Ghuman J. et al. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353, 38–52, doi: S0022-2836(05)00885-5 (2005). - PubMed
-
- Troiber C. et al. Stabilizing effect of tyrosine trimers on pDNA and siRNA polyplexes. Biomaterials 34, 1624–1633, doi: S0142-9612(12)01265-3 (2013). - PubMed
-
- Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release 132, 171–183, doi: S0168-3659(08)00255-1 (2008). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

