Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells
- PMID: 26658786
- PMCID: PMC4676010
- DOI: 10.1038/srep18046
Mitotic Spindle Disruption by Alternating Electric Fields Leads to Improper Chromosome Segregation and Mitotic Catastrophe in Cancer Cells
Abstract
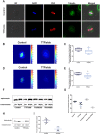
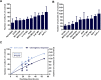
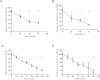
Tumor Treating Fields (TTFields) are low intensity, intermediate frequency, alternating electric fields. TTFields are a unique anti-mitotic treatment modality delivered in a continuous, noninvasive manner to the region of a tumor. It was previously postulated that by exerting directional forces on highly polar intracellular elements during mitosis, TTFields could disrupt the normal assembly of spindle microtubules. However there is limited evidence directly linking TTFields to an effect on microtubules. Here we report that TTFields decrease the ratio between polymerized and total tubulin, and prevent proper mitotic spindle assembly. The aberrant mitotic events induced by TTFields lead to abnormal chromosome segregation, cellular multinucleation, and caspase dependent apoptosis of daughter cells. The effect of TTFields on cell viability and clonogenic survival substantially depends upon the cell division rate. We show that by extending the duration of exposure to TTFields, slowly dividing cells can be affected to a similar extent as rapidly dividing cells.
Conflict of interest statement
We wish to disclose that Yoram Palti holds stock in Novocure Ltd. All other authors, except Yoram Palti are paid employees of Novocure. There are no other known conflicts of interest associated with this publication.
Figures




References
-
- McCaig C. D., Rajnicek A. M., Song B. & Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol. Rev. 85, 943–978 (2005). - PubMed
-
- Bassett C. A. The development and application of pulsed electromagnetic fields (PEMFs) for ununited fractures and arthrodeses. Clin. Plast. Surg. 12, 259–277 (1985). - PubMed
-
- Repacholi M. H. & Greenebaum B. Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics 20, 133–160 (1999). - PubMed
-
- Elson E. Biologic effects of radiofrequency and microwave fields: in vivo and in vitro experimental results in The biomedical engineering handbook (ed. Bronzino J. D.) 1417–1423 (CRC Press, 1995).
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

