Clinical, Virologic, Immunologic Outcomes and Emerging HIV Drug Resistance Patterns in Children and Adolescents in Public ART Care in Zimbabwe
- PMID: 26658814
- PMCID: PMC4678607
- DOI: 10.1371/journal.pone.0144057
Clinical, Virologic, Immunologic Outcomes and Emerging HIV Drug Resistance Patterns in Children and Adolescents in Public ART Care in Zimbabwe
Abstract
Objective: To determine immunologic, virologic outcomes and drug resistance among children and adolescents receiving care during routine programmatic implementation in a low-income country.
Methods: A cross-sectional evaluation with collection of clinical and laboratory data for children (0-<10 years) and adolescents (10-19 years) attending a public ART program in Harare providing care for pediatric patients since 2004, was conducted. Longitudinal data for each participant was obtained from the clinic based medical record.
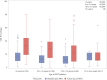
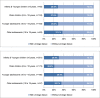
Results: Data from 599 children and adolescents was evaluated. The participants presented to care with low CD4 cell count and CD4%, median baseline CD4% was lower in adolescents compared with children (11.0% vs. 15.0%, p<0.0001). The median age at ART initiation was 8.0 years (IQR 3.0, 12.0); median time on ART was 2.9 years (IQR 1.7, 4.5). On ART, median CD4% improved for all age groups but remained below 25%. Older age (≥ 5 years) at ART initiation was associated with severe stunting (HAZ <-2: 53.3% vs. 28.4%, p<0.0001). Virologic failure rate was 30.6% and associated with age at ART initiation. In children, nevirapine based ART regimen was associated with a 3-fold increased risk of failure (AOR: 3.5; 95% CI: 1.3, 9.1, p = 0.0180). Children (<10 y) on ART for ≥4 years had higher failure rates than those on ART for <4 years (39.6% vs. 23.9%, p = 0.0239). In those initiating ART as adolescents, each additional year in age above 10 years at the time of ART initiation (AOR 0.4 95%CI: 0.1, 0.9, p = 0.0324), and each additional year on ART (AOR 0.4, 95%CI 0.2, 0.9, p = 0.0379) were associated with decreased risk of virologic failure. Drug resistance was evident in 67.6% of sequenced virus isolates.
Conclusions: During routine programmatic implementation of HIV care for children and adolescents, delayed age at ART initiation has long-term implications on immunologic recovery, growth and virologic outcomes.
Conflict of interest statement
Figures
Similar articles
-
Community Based Antiretroviral Treatment in Rural Zimbabwe.AIDS Res Hum Retroviruses. 2017 Dec;33(12):1185-1191. doi: 10.1089/aid.2017.0029. Epub 2017 Oct 26. AIDS Res Hum Retroviruses. 2017. PMID: 28899102
-
Effectiveness of first-line antiretroviral therapy and correlates of longitudinal changes in CD4 and viral load among HIV-infected children in Ghana.BMC Infect Dis. 2013 Oct 13;13:476. doi: 10.1186/1471-2334-13-476. BMC Infect Dis. 2013. PMID: 24119088 Free PMC article.
-
Long-term virological outcome in children receiving first-line antiretroviral therapy.AIDS Res Ther. 2018 Nov 26;15(1):23. doi: 10.1186/s12981-018-0208-9. AIDS Res Ther. 2018. PMID: 30477526 Free PMC article.
-
Current ART, determinants for virologic failure and implications for HIV drug resistance: an umbrella review.AIDS Res Ther. 2023 Oct 27;20(1):74. doi: 10.1186/s12981-023-00572-6. AIDS Res Ther. 2023. PMID: 37884997 Free PMC article.
-
Treatment of HIV Infection in Children Across the Age Spectrum: Achievements and New Prospects.Clin Perinatol. 2024 Dec;51(4):817-832. doi: 10.1016/j.clp.2024.08.003. Epub 2024 Sep 25. Clin Perinatol. 2024. PMID: 39487022 Review.
Cited by
-
A population pharmacokinetic model is beneficial in quantifying hair concentrations of ritonavir-boosted atazanavir: a study of HIV-infected Zimbabwean adolescents.BMC Pharmacol Toxicol. 2020 Aug 3;21(1):58. doi: 10.1186/s40360-020-00437-y. BMC Pharmacol Toxicol. 2020. PMID: 32746923 Free PMC article. Clinical Trial.
-
Immunological failure in HIV-infected adults from 2003 to 2015 in Southwest Ethiopia: a retrospective cohort study.BMJ Open. 2018 Aug 17;8(8):e017413. doi: 10.1136/bmjopen-2017-017413. BMJ Open. 2018. PMID: 30121586 Free PMC article.
-
Patterns of detectable viral load in a cohort of HIV-positive adolescents on antiretroviral therapy in South Africa.J Int AIDS Soc. 2020 Mar;23(3):e25474. doi: 10.1002/jia2.25474. J Int AIDS Soc. 2020. PMID: 32180367 Free PMC article.
-
Virological Outcomes Among Pregnant Women Receiving Antiretroviral Treatment in the Amhara Region, North West Ethiopia.HIV AIDS (Auckl). 2023 May 2;15:209-216. doi: 10.2147/HIV.S389506. eCollection 2023. HIV AIDS (Auckl). 2023. PMID: 37159581 Free PMC article.
-
Evaluating a multi-component, community-based program to improve adherence and retention in care among adolescents living with HIV in Zimbabwe: study protocol for a cluster randomized controlled trial.Trials. 2017 Oct 20;18(1):478. doi: 10.1186/s13063-017-2198-7. Trials. 2017. PMID: 29052529 Free PMC article. Clinical Trial.
References
-
- WHO (2013) Global update on HIV treatment 2013: results, impact and opportunities: World Health Organization report in partnership with UNICEF and UNAIDS. http://www.who.int/hiv/pub/progressreports/update2013/en/.
-
- WHO (2013) Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV infection. Recommendations for a public health approach. http://wwwwhoint/hiv/pub/guidelines/arv2013/download/en/. - PubMed
-
- WHO (2015) Guideline on when to Start Antiretroviral Therapy and on Pre-Exposure Prophylaxis for HIV. http://www.who.int/hiv/pub/guidelines/earlyrelease-arv/en/: WHO. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials