MEG Multivariate Analysis Reveals Early Abstract Action Representations in the Lateral Occipitotemporal Cortex
- PMID: 26658857
- PMCID: PMC6605497
- DOI: 10.1523/JNEUROSCI.1422-15.2015
MEG Multivariate Analysis Reveals Early Abstract Action Representations in the Lateral Occipitotemporal Cortex
Abstract
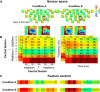
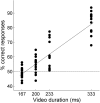
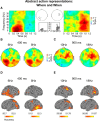
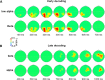
Understanding other people's actions is a fundamental prerequisite for social interactions. Whether action understanding relies on simulating the actions of others in the observers' motor system or on the access to conceptual knowledge stored in nonmotor areas is strongly debated. It has been argued previously that areas that play a crucial role in action understanding should (1) distinguish between different actions, (2) generalize across the ways in which actions are performed (Dinstein et al., 2008; Oosterhof et al., 2013; Caramazza et al., 2014), and (3) have access to action information around the time of action recognition (Hauk et al., 2008). Whereas previous studies focused on the first two criteria, little is known about the dynamics underlying action understanding. We examined which human brain regions are able to distinguish between pointing and grasping, regardless of reach direction (left or right) and effector (left or right hand), using multivariate pattern analysis of magnetoencephalography data. We show that the lateral occipitotemporal cortex (LOTC) has the earliest access to abstract action representations, which coincides with the time point from which there was enough information to allow discriminating between the two actions. By contrast, precentral regions, though recruited early, have access to such abstract representations substantially later. Our results demonstrate that in contrast to the LOTC, the early recruitment of precentral regions does not contain the detailed information that is required to recognize an action. We discuss previous theoretical claims of motor theories and how they are incompatible with our data.
Significance statement: It is debated whether our ability to understand other people's actions relies on the simulation of actions in the observers' motor system, or is based on access to conceptual knowledge stored in nonmotor areas. Here, using magnetoencephalography in combination with machine learning, we examined where in the brain and at which point in time it is possible to distinguish between pointing and grasping actions regardless of the way in which they are performed (effector, reach direction). We show that, in contrast to the predictions of motor theories of action understanding, the lateral occipitotemporal cortex has access to abstract action representations substantially earlier than precentral regions.
Keywords: MVPA; action observation; action understanding; neural dynamics.
Copyright © 2015 the authors 0270-6474/15/3516034-12$15.00/0.
Figures





Similar articles
-
Action Categories in Lateral Occipitotemporal Cortex Are Organized Along Sociality and Transitivity.J Neurosci. 2017 Jan 18;37(3):562-575. doi: 10.1523/JNEUROSCI.1717-16.2016. J Neurosci. 2017. PMID: 28100739 Free PMC article.
-
MEG adaptation reveals action representations in posterior occipitotemporal regions.Cortex. 2018 Jun;103:266-276. doi: 10.1016/j.cortex.2018.03.016. Epub 2018 Mar 27. Cortex. 2018. PMID: 29673783
-
Surface-based information mapping reveals crossmodal vision-action representations in human parietal and occipitotemporal cortex.J Neurophysiol. 2010 Aug;104(2):1077-89. doi: 10.1152/jn.00326.2010. Epub 2010 Jun 10. J Neurophysiol. 2010. PMID: 20538772 Free PMC article.
-
The lateral occipitotemporal cortex in action.Trends Cogn Sci. 2015 May;19(5):268-77. doi: 10.1016/j.tics.2015.03.006. Epub 2015 Apr 3. Trends Cogn Sci. 2015. PMID: 25843544 Review.
-
Body selectivity in occipitotemporal cortex: Causal evidence.Neuropsychologia. 2016 Mar;83:138-148. doi: 10.1016/j.neuropsychologia.2015.05.033. Epub 2015 Jun 2. Neuropsychologia. 2016. PMID: 26044771 Review.
Cited by
-
Dyadic interaction processing in the posterior temporal cortex.Neuroimage. 2019 Sep;198:296-302. doi: 10.1016/j.neuroimage.2019.05.027. Epub 2019 May 14. Neuroimage. 2019. PMID: 31100434 Free PMC article.
-
Crossmodal Classification of Mu Rhythm Activity during Action Observation and Execution Suggests Specificity to Somatosensory Features of Actions.J Neurosci. 2017 Jun 14;37(24):5936-5947. doi: 10.1523/JNEUROSCI.3393-16.2017. Epub 2017 May 30. J Neurosci. 2017. PMID: 28559380 Free PMC article.
-
Dopaminergic modulation of behavioral and electrocortical markers of interpersonal performance monitoring in Parkinson's Disease.Commun Biol. 2025 Aug 12;8(1):1195. doi: 10.1038/s42003-025-08638-z. Commun Biol. 2025. PMID: 40797097 Free PMC article.
-
Decoding across sensory modalities reveals common supramodal signatures of conscious perception.Proc Natl Acad Sci U S A. 2020 Mar 31;117(13):7437-7446. doi: 10.1073/pnas.1912584117. Epub 2020 Mar 17. Proc Natl Acad Sci U S A. 2020. PMID: 32184331 Free PMC article.
-
Action Categories in Lateral Occipitotemporal Cortex Are Organized Along Sociality and Transitivity.J Neurosci. 2017 Jan 18;37(3):562-575. doi: 10.1523/JNEUROSCI.1717-16.2016. J Neurosci. 2017. PMID: 28100739 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
