Re-Opening the Critical Window for Estrogen Therapy
- PMID: 26658861
- PMCID: PMC4682778
- DOI: 10.1523/JNEUROSCI.1890-15.2015
Re-Opening the Critical Window for Estrogen Therapy
Abstract
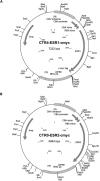
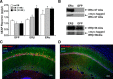
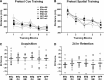
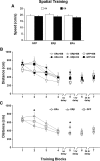
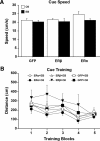
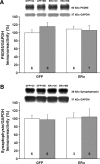
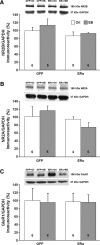
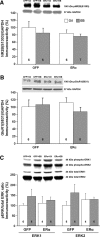
A decline in estradiol (E2)-mediated cognitive benefits denotes a critical window for the therapeutic effects of E2, but the mechanism for closing of the critical window is unknown. We hypothesized that upregulating the expression of estrogen receptor α (ERα) or estrogen receptor β (ERβ) in the hippocampus of aged animals would restore the therapeutic potential of E2 treatments and rejuvenate E2-induced hippocampal plasticity. Female rats (15 months) were ovariectomized, and, 14 weeks later, adeno-associated viral vectors were used to express ERα, ERβ, or green fluorescent protein (GFP) in the CA1 region of the dorsal hippocampus. Animals were subsequently treated for 5 weeks with cyclic injections of 17β-estradiol-3-benzoate (EB, 10 μg) or oil vehicle. Spatial memory was examined 48 h after EB/oil treatment. EB treatment in the GFP (GFP + EB) and ERβ (ERβ + EB) groups failed to improve episodic spatial memory relative to oil-treated animals, indicating closing of the critical window. Expression of ERβ failed to improve cognition and was associated with a modest learning impairment. Cognitive benefits were specific to animals expressing ERα that received EB treatment (ERα + EB), such that memory was improved relative to ERα + oil and GFP + EB. Similarly, ERα + EB animals exhibited enhanced NMDAR-mediated synaptic transmission compared with the ERα + oil and GFP + EB groups. This is the first demonstration that the window for E2-mediated benefits on cognition and hippocampal E2 responsiveness can be reinstated by increased expression of ERα.
Significance statement: Estradiol is neuroprotective, promotes synaptic plasticity in the hippocampus, and protects against cognitive decline associated with aging and neurodegenerative diseases. However, animal models and clinical studies indicate a critical window for the therapeutic treatment such that the beneficial effects are lost with advanced age and/or with extended hormone deprivation. We used gene therapy to upregulate expression of the estrogen receptors ERα and ERβ and demonstrate that the window for estradiol's beneficial effects on memory and hippocampal synaptic function can be reinstated by enhancing the expression of ERα. Our findings suggest that the activity of ERα controls the therapeutic window by regulating synaptic plasticity mechanisms involved in memory.
Keywords: ERα and ERβ; NMDA receptor; aging; estrogen; hippocampus; learning and memory.
Copyright © 2015 the authors 0270-6474/15/3516077-17$15.00/0.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
