Variation in Screening Abnormality Rates and Follow-Up of Breast, Cervical and Colorectal Cancer Screening within the PROSPR Consortium
- PMID: 26658934
- PMCID: PMC4803707
- DOI: 10.1007/s11606-015-3552-7
Variation in Screening Abnormality Rates and Follow-Up of Breast, Cervical and Colorectal Cancer Screening within the PROSPR Consortium
Abstract
Background: Primary care providers and health systems have prominent roles in guiding effective cancer screening.
Objective: To characterize variation in screening abnormality rates and timely initial follow-up for common cancer screening tests.
Design: Population-based cohort undergoing screening in 2011, 2012, or 2013 at seven research centers comprising the National Cancer Institute-sponsored Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium.
Participants: Adults undergoing mammography with or without digital breast tomosynthesis (n = 97,683 ages 40-75 years), fecal occult blood or fecal immunochemical tests (n = 759,553 ages 50-75 years), or Papanicolaou with or without human papillomavirus tests (n = 167,330 ages 21-65 years).
Intervention: Breast, colorectal, or cervical cancer screening.
Main measures: Abnormality rates per 1000 screens; percentage with timely initial follow-up (within 90 days, except 9-month window for BI-RADS 3). Primary care clinic-level variation in percentage with screening abnormality and percentage with timely initial follow-up.
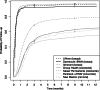
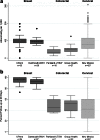
Key results: There were 10,248/97,683 (104.9 per 1000) abnormal breast cancer screens, 35,847/759,553 (47.2 per 1000) FOBT/FIT-positive colorectal cancer screens, and 13,266/167,330 (79.3 per 1000) abnormal cervical cancer screens. The percentage with timely follow-up was 93.2 to 96.7 % for breast centers, 46.8 to 68.7 % for colorectal centers, and 46.6 % for the cervical cancer screening center (low-grade squamous intraepithelial lesions or higher). The primary care clinic variation (25th to 75th percentile) was smaller for the percentage with an abnormal screen (breast, 8.5-10.3 %; colorectal, 3.0-4.8 %; cervical, 6.3-9.9 %) than for the percentage with follow-up within 90 days (breast, 90.2-95.8 %; colorectal, 43.4-52.0 %; cervical, 29.6-61.4 %).
Conclusions: Variation in both the rate of screening abnormalities and their initial follow-up was evident across organ sites and primary care clinics. This highlights an opportunity for improving the delivery of cancer screening through focused study of patient, provider, clinic, and health system characteristics associated with timely follow-up of screening abnormalities.
Keywords: breast cancer screening; cervical cancer screening; colorectal cancer screening; practice variation.
Conflict of interest statement
The authors declare no conflicts of interest. Funders This work was supported by the National Cancer Institute (NCI)-funded Population-based Research Optimizing Screening through Personalized Regimens (PROSPR) consortium (grant numbers U01CA163304 to M.T., W.B.; U54CA163303 to D.L.W., B.S.; U54CA163307 to A.N.A.T., T.O., J.H.; U54CA163313 to K.A., M.S.; U54CA163308 to C.S.S., E.H.; U54CA163308-04S1 to C.S.S., J.A.T.; U54CA163261 to C.R.; U54CA163261-04S1 to J.C., A.K.; U54CA163262 to A.G.Z., D.C., C.D., T.L.; U54CA163262-04S1 to D.C., M.S.; and U54CA164336 to C.W.). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Veterans Affairs or the United States government.
Figures
Comment in
-
Capsule Commentary on Tosteson et al., Variation in Screening Abnormality Rates and Follow-Up of Breast, Cervical and Colorectal Cancer Screening within the PROSPR Consortium.J Gen Intern Med. 2016 Apr;31(4):411. doi: 10.1007/s11606-016-3594-5. J Gen Intern Med. 2016. PMID: 26857730 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
- U54CA163303/CA/NCI NIH HHS/United States
- P30 CA118100/CA/NCI NIH HHS/United States
- U54CA163307/CA/NCI NIH HHS/United States
- U54CA163262-04S1/CA/NCI NIH HHS/United States
- U54CA163262/CA/NCI NIH HHS/United States
- UL1 TR001105/TR/NCATS NIH HHS/United States
- U54 CA163307/CA/NCI NIH HHS/United States
- U54CA163261-04S1/CA/NCI NIH HHS/United States
- P30 CA023108/CA/NCI NIH HHS/United States
- U54CA163308/CA/NCI NIH HHS/United States
- U54CA164336/CA/NCI NIH HHS/United States
- U01CA163304/CA/NCI NIH HHS/United States
- U54CA163313/CA/NCI NIH HHS/United States
- U54CA163308-04S1/CA/NCI NIH HHS/United States
- U54 CA163308/CA/NCI NIH HHS/United States
- U54CA163261/CA/NCI NIH HHS/United States
- U54 CA164336/CA/NCI NIH HHS/United States
- U01 CA163304/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

