Establishment of an Algorithm Using prM/E- and NS1-Specific IgM Antibody-Capture Enzyme-Linked Immunosorbent Assays in Diagnosis of Japanese Encephalitis Virus and West Nile Virus Infections in Humans
- PMID: 26659204
- PMCID: PMC4733197
- DOI: 10.1128/JCM.02469-15
Establishment of an Algorithm Using prM/E- and NS1-Specific IgM Antibody-Capture Enzyme-Linked Immunosorbent Assays in Diagnosis of Japanese Encephalitis Virus and West Nile Virus Infections in Humans
Abstract
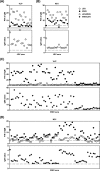
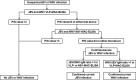
The front-line assay for the presumptive serodiagnosis of acute Japanese encephalitis virus (JEV) and West Nile virus (WNV) infections is the premembrane/envelope (prM/E)-specific IgM antibody-capture enzyme-linked immunosorbent assay (MAC-ELISA). Due to antibody cross-reactivity, MAC-ELISA-positive samples may be confirmed with a time-consuming plaque reduction neutralization test (PRNT). In the present study, we applied a previously developed anti-nonstructural protein 1 (NS1)-specific MAC-ELISA (NS1-MAC-ELISA) on archived acute-phase serum specimens from patients with confirmed JEV and WNV infections and compared the results with prM/E containing virus-like particle-specific MAC-ELISA (VLP-MAC-ELISA). Paired-receiver operating characteristic (ROC) curve analyses revealed no statistical differences in the overall assay performances of the VLP- and NS1-MAC-ELISAs. The two methods had high sensitivities of 100% but slightly lower specificities that ranged between 80% and 100%. When the NS1-MAC-ELISA was used to confirm positive results in the VLP-MAC-ELISA, the specificity of serodiagnosis, especially for JEV infection, was increased to 90% when applied in areas where JEV cocirculates with WNV, or to 100% when applied in areas that were endemic for JEV. The results also showed that using multiple antigens could resolve the cross-reactivity in the assays. Significantly higher positive-to-negative (P/N) values were consistently obtained with the homologous antigens than those with the heterologous antigens. JEV or WNV was reliably identified as the currently infecting flavivirus by a higher ratio of JEV-to-WNV P/N values or vice versa. In summary of the above-described results, the diagnostic algorithm combining the use of multiantigen VLP- and NS1-MAC-ELISAs was developed and can be practically applied to obtain a more specific and reliable result for the serodiagnosis of JEV and WNV infections without the need for PRNT. The developed algorithm should provide great utility in diagnostic and surveillance activities in which test accuracy is of utmost importance for effective disease intervention.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures



References
-
- Lindenbach BD, Murray CL, Thiel HL, Rice CM. 2013. Flaviviridae: the viruses and their replication, p 712–746. In Knipe DM, Howley PM (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Lwande OW, Mosomtai G, Symekher S. 2015. West Nile virus, a reemerging virus. Precis Med 2:e604. doi:10.14800/pm.604. - DOI
-
- Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH, Hall RA, MacKenzie JS, Cropp CB, Panigrahy B, Ostlund E, Schmitt B, Malkinson M, Banet C, Weissman J, Komar N, Savage HM, Stone W, McNamara T, Gubler DJ. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science 286:2333–2337. doi:10.1126/science.286.5448.2333. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

