A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys
- PMID: 26659273
- PMCID: PMC4675964
- DOI: 10.1038/srep17943
A novel, native-format bispecific antibody triggering T-cell killing of B-cells is robustly active in mouse tumor models and cynomolgus monkeys
Abstract
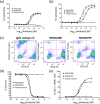
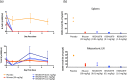
Bispecific antibodies, while showing great therapeutic potential, pose formidable challenges with respect to their assembly, stability, immunogenicity, and pharmacodynamics. Here we describe a novel class of bispecific antibodies with native human immunoglobulin format. The design exploits differences in the affinities of the immunoglobulin isotypes for Protein A, allowing efficient large-scale purification. Using this format, we generated a bispecific antibody, REGN1979, targeting the B cell marker, CD20, and the CD3 component of the T cell receptor, which triggers redirected killing of B cells. In mice, this antibody prevented growth of B cell tumors and also caused regression of large established tumors. In cynomolgus monkeys, low doses of REGN1979 caused prolonged depletion of B cells in peripheral blood with a serum half-life of approximately 14 days. Further, the antibody induced a deeper depletion of B cells in lymphoid organs than rituximab. This format has broad applicability for development of clinical bispecific antibodies.
Conflict of interest statement
There is potential competing interest. All authors are employees of Regeneron Phamaceuticals.
Figures





References
-
- Spiess C., Zhai Q. & Carter P. J. Alternative molecular formats and therapeutic applications for bispecific antibodies. Mol. Immunol. 67, 95–106 (2015). - PubMed
-
- Ridgway J. B., Presta L. G. & Carter P. ‘Knobs-into-holes’ engineering of antibody CH3 domains for heavy chain heterodimerization. Protein Eng. 9, 617–21 (1996). - PubMed
-
- Lindhofer H., Mocikat R., Steipe B. & Thierfelder S. Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies. J. Immunol. 155, 219–25 (1995). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

