Cortical Network Models of Firing Rates in the Resting and Active States Predict BOLD Responses
- PMID: 26659399
- PMCID: PMC4678290
- DOI: 10.1371/journal.pone.0144796
Cortical Network Models of Firing Rates in the Resting and Active States Predict BOLD Responses
Abstract
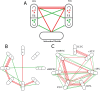
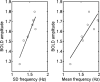
Measurements of blood oxygenation level dependent (BOLD) signals have produced some surprising observations. One is that their amplitude is proportional to the entire activity in a region of interest and not just the fluctuations in this activity. Another is that during sleep and anesthesia the average BOLD correlations between regions of interest decline as the activity declines. Mechanistic explanations of these phenomena are described here using a cortical network model consisting of modules with excitatory and inhibitory neurons, taken as regions of cortical interest, each receiving excitatory inputs from outside the network, taken as subcortical driving inputs in addition to extrinsic (intermodular) connections, such as provided by associational fibers. The model shows that the standard deviation of the firing rate is proportional to the mean frequency of the firing when the extrinsic connections are decreased, so that the mean BOLD signal is proportional to both as is observed experimentally. The model also shows that if these extrinsic connections are decreased or the frequency of firing reaching the network from the subcortical driving inputs is decreased, or both decline, there is a decrease in the mean firing rate in the modules accompanied by decreases in the mean BOLD correlations between the modules, consistent with the observed changes during NREM sleep and under anesthesia. Finally, the model explains why a transient increase in the BOLD signal in a cortical area, due to a transient subcortical input, gives rises to responses throughout the cortex as observed, with these responses mediated by the extrinsic (intermodular) connections.
Conflict of interest statement
Figures



Similar articles
-
Cortical network models of impulse firing in the resting and active states predict cortical energetics.Proc Natl Acad Sci U S A. 2015 Mar 31;112(13):4134-9. doi: 10.1073/pnas.1411513112. Epub 2015 Mar 16. Proc Natl Acad Sci U S A. 2015. PMID: 25775588 Free PMC article.
-
On the origins of the 'global signal' determined by functional magnetic resonance imaging in the resting state.J Neural Eng. 2016 Feb;13(1):016012. doi: 10.1088/1741-2560/13/1/016012. Epub 2015 Dec 17. J Neural Eng. 2016. PMID: 26678535
-
Cortical network modeling: analytical methods for firing rates and some properties of networks of LIF neurons.J Physiol Paris. 2006 Jul-Sep;100(1-3):88-99. doi: 10.1016/j.jphysparis.2006.09.001. Epub 2006 Oct 24. J Physiol Paris. 2006. PMID: 17064883
-
Intracellular and computational evidence for a dominant role of internal network activity in cortical computations.Curr Opin Neurobiol. 2011 Oct;21(5):717-25. doi: 10.1016/j.conb.2011.06.002. Epub 2011 Jun 27. Curr Opin Neurobiol. 2011. PMID: 21715156 Review.
-
Neuronal inhibition and excitation, and the dichotomic control of brain hemodynamic and oxygen responses.Neuroimage. 2012 Aug 15;62(2):1040-50. doi: 10.1016/j.neuroimage.2012.01.040. Epub 2012 Jan 12. Neuroimage. 2012. PMID: 22261372 Review.
Cited by
-
BOLD Monitoring in the Neural Simulator ANNarchy.Front Neuroinform. 2022 Mar 22;16:790966. doi: 10.3389/fninf.2022.790966. eCollection 2022. Front Neuroinform. 2022. PMID: 35392282 Free PMC article.
References
-
- Colonnese MT, Phillips MA, Constantine-Paton M, Kaila K, Jasanoff A. Development of hemodynamic responses and functional connectivity in rat somatosensory cortex. Nat Neurosci. 2008;11(1):72–9. Epub 2007 Nov 25. - PubMed
-
- Biswal BB, Van Kylen J, Hyde JS. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 1997;10(4–5):165–70. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

