Pharmacokinetics and pharmacodynamics of γ-hydroxybutyrate in healthy subjects
- PMID: 26659543
- PMCID: PMC4834605
- DOI: 10.1111/bcp.12863
Pharmacokinetics and pharmacodynamics of γ-hydroxybutyrate in healthy subjects
Abstract
Aims: γ-Hydroxybutyrate (GHB) is used as a treatment for narcolepsy and alcohol withdrawal and as a recreational substance. Nevertheless, there are limited data on the pharmacokinetics and pharmacokinetic-pharmacodynamic relationships of GHB in humans. We characterized the pharmacokinetic profile and exposure-psychotropic effect relationship of GHB in humans.
Methods: Two oral doses of GHB (25 and 35 mg kg(-1) ) were administered to 32 healthy male subjects (16 for each dose) using a randomized, placebo-controlled, cross-over design.
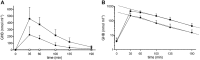
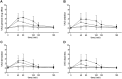
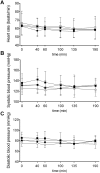
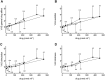
Results: Maximal concentrations of GHB were (geometric mean and 95% CI): 218 (176-270) nmol ml(-1) and 453 (374-549) nmol ml(-1) for the 25 and 35 mg kg(-1) GHB doses, respectively. The elimination half-lives (mean ± SD) were 36 ± 9 and 39 ± 7 min and the AUC∞ values (geometric mean and 95% CI) were 15 747 (12 854-19 290) and 40 113 (33 093-48 622) nmol∙min ml(-1) for the 20 and 35 mg kg(-1) GHB doses, respectively. Thus, plasma GHB exposure (AUC0-∞ ) rose disproportionally (+40%) with the higher dose. γ-Hydroxybutyrate produced mixed stimulant-sedative effects, with a dose-dependent increase in sedation and dizziness. It did not alter heart rate or blood pressure. A close relationship between plasma GHB exposure and its psychotropic effects was found, with higher GHB concentrations associated with higher subjective stimulation, sedation, and dizziness. No clockwise hysteresis was observed in the GHB concentration effect plot over time (i.e., no acute pharmacological tolerance).
Conclusion: Evidence was found of a nonlinear dose-exposure relationship (i.e., no dose proportionality) at moderate doses of GHB. The effects of GHB on consciousness were closely linked to its plasma exposure and exhibited no acute tolerance.
Keywords: GHB; liquid ecstasy; pharmacokinetic; sodium oxybate.
© 2015 The British Pharmacological Society.
Figures
References
-
- Snead OC III. Evidence for a G protein‐coupled γ‐hydroxybutyric acid receptor. J Neurochem 2000; 75: 1986–96. - PubMed
-
- Hu RQ, Banerjee PK, Snead OC III. Regulation of γ‐aminobutyric acid (GABA) release in cerebral cortex in the γ‐hydroxybutyric acid (GHB) model of absence seizures in rat. Neuropharmacology 2000; 39: 427–39. - PubMed
-
- Wong CG, Gibson KM, Snead OC III. From the street to the brain: neurobiology of the recreational drug γ‐hydroxybutyric acid. Trends Pharmacol Sci 2004; 25: 29–34. - PubMed
-
- Chin RL, Sporer KA, Cullison B, Dyer JE, Wu TD. Clinical course of γ‐hydroxybutyrate overdose. Ann Emerg Med 1998; 31: 716–22. - PubMed
-
- Liechti ME, Kupferschmidt H. Gamma‐hydroxybutyrate (GHB) and gamma‐butyrolactone (GBL): analysis of overdose cases reported to the Swiss toxicological information centre. Swiss Med Wkly 2004; 134: 534–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

