Neurotropic virus infections as the cause of immediate and delayed neuropathology
- PMID: 26659576
- PMCID: PMC4713712
- DOI: 10.1007/s00401-015-1511-3
Neurotropic virus infections as the cause of immediate and delayed neuropathology
Abstract
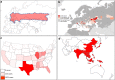
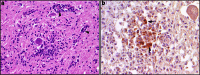
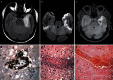
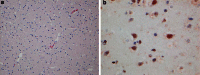
A wide range of viruses from different virus families in different geographical areas, may cause immediate or delayed neuropathological changes and neurological manifestations in humans and animals. Infection by neurotropic viruses as well as the resulting immune response can irreversibly disrupt the complex structural and functional architecture of the central nervous system, frequently leaving the patient or affected animal with a poor or fatal prognosis. Mechanisms that govern neuropathogenesis and immunopathogenesis of viral infections are highlighted, using examples of well-studied virus infections that are associated with these alterations in different populations throughout the world. A better understanding of the molecular, epidemiological and biological characteristics of these infections and in particular of mechanisms that underlie their clinical manifestations may be expected to provide tools for the development of more effective intervention strategies and treatment regimens.
Keywords: Alphavirus; Bornavirus; Bunyavirus; Central nervous system; Flavivirus; Herpesvirus; Influenza virus; Neuroinfectiology; Neuropathology; Paramyxovirus; Picornavirus; Rhabdovirus; Virus infection.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

