Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy
- PMID: 26659578
- PMCID: PMC4879001
- DOI: 10.1007/s00401-015-1509-x
Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy
Abstract
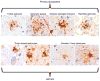
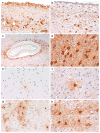
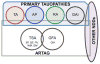
Pathological accumulation of abnormally phosphorylated tau protein in astrocytes is a frequent, but poorly characterized feature of the aging brain. Its etiology is uncertain, but its presence is sufficiently ubiquitous to merit further characterization and classification, which may stimulate clinicopathological studies and research into its pathobiology. This paper aims to harmonize evaluation and nomenclature of aging-related tau astrogliopathy (ARTAG), a term that refers to a morphological spectrum of astroglial pathology detected by tau immunohistochemistry, especially with phosphorylation-dependent and 4R isoform-specific antibodies. ARTAG occurs mainly, but not exclusively, in individuals over 60 years of age. Tau-immunoreactive astrocytes in ARTAG include thorn-shaped astrocytes at the glia limitans and in white matter, as well as solitary or clustered astrocytes with perinuclear cytoplasmic tau immunoreactivity that extends into the astroglial processes as fine fibrillar or granular immunopositivity, typically in gray matter. Various forms of ARTAG may coexist in the same brain and might reflect different pathogenic processes. Based on morphology and anatomical distribution, ARTAG can be distinguished from primary tauopathies, but may be concurrent with primary tauopathies or other disorders. We recommend four steps for evaluation of ARTAG: (1) identification of five types based on the location of either morphologies of tau astrogliopathy: subpial, subependymal, perivascular, white matter, gray matter; (2) documentation of the regional involvement: medial temporal lobe, lobar (frontal, parietal, occipital, lateral temporal), subcortical, brainstem; (3) documentation of the severity of tau astrogliopathy; and (4) description of subregional involvement. Some types of ARTAG may underlie neurological symptoms; however, the clinical significance of ARTAG is currently uncertain and awaits further studies. The goal of this proposal is to raise awareness of astroglial tau pathology in the aged brain, facilitating communication among neuropathologists and researchers, and informing interpretation of clinical biomarkers and imaging studies that focus on tau-related indicators.
Keywords: ARTAG; Aging; Tau; Tau astrogliopathy.
Figures

References
-
- Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, et al. Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol. 2009;117:635–652. - PubMed
-
- Arima K, Izumiyama Y, Nakamura M, Nakayama H, Kimura M, Ando S, Ikeda K, Takahashi K. Argyrophilic tau-positive twisted and non-twisted tubules in astrocytic processes in brains of Alzheimer-type dementia: an electron microscopical study. Acta Neuropathol. 1998;95:28–39. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50 AG05136/AG/NIA NIH HHS/United States
- P30 AG010133/AG/NIA NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- P01 AG019724-03/AG/NIA NIH HHS/United States
- AG010124/AG/NIA NIH HHS/United States
- G0900580/MRC_/Medical Research Council/United Kingdom
- R01 NS095252/NS/NINDS NIH HHS/United States
- ARC_/Arthritis Research UK/United Kingdom
- P50 AG005138/AG/NIA NIH HHS/United States
- R28 AA012725/AA/NIAAA NIH HHS/United States
- G0901945/MRC_/Medical Research Council/United Kingdom
- P50AG05146/AG/NIA NIH HHS/United States
- P30 AG028383/AG/NIA NIH HHS/United States
- P30AG008051-26/AG/NIA NIH HHS/United States
- P30 AG013854/AG/NIA NIH HHS/United States
- G0900582/MRC_/Medical Research Council/United Kingdom
- MR/L016451/1/MRC_/Medical Research Council/United Kingdom
- P50 AG05681/AG/NIA NIH HHS/United States
- P30 AG010124/AG/NIA NIH HHS/United States
- P50 AG023501/AG/NIA NIH HHS/United States
- G1100540/MRC_/Medical Research Council/United Kingdom
- P30 AG10133/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- P50 AG005146/AG/NIA NIH HHS/United States
- P01 AG017586/AG/NIA NIH HHS/United States
- AG017586/AG/NIA NIH HHS/United States
- R01 AG040311/AG/NIA NIH HHS/United States
- AG028383/AG/NIA NIH HHS/United States
- U01 NS086659/NS/NINDS NIH HHS/United States
- MR/L016400/1/MRC_/Medical Research Council/United Kingdom
- P01 AG003991/AG/NIA NIH HHS/United States
- P30 AG008051/AG/NIA NIH HHS/United States
- P50 AG005681/AG/NIA NIH HHS/United States
- P30AG12300/AG/NIA NIH HHS/United States
- P01 AG03991/AG/NIA NIH HHS/United States
- R01 NS094003/NS/NINDS NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- P30 AG012300/AG/NIA NIH HHS/United States
- G0900652/MRC_/Medical Research Council/United Kingdom
- P01 AG019724/AG/NIA NIH HHS/United States
- G0502157/MRC_/Medical Research Council/United Kingdom
- G0400074/MRC_/Medical Research Council/United Kingdom
- P50 NS072187/NS/NINDS NIH HHS/United States
- P50 AG005133/AG/NIA NIH HHS/United States
- P50 NS062684/NS/NINDS NIH HHS/United States
- AG13854/AG/NIA NIH HHS/United States
- 1U01NS086659-01/NS/NINDS NIH HHS/United States
- P30AG13846/AG/NIA NIH HHS/United States
- G0600953/MRC_/Medical Research Council/United Kingdom
- MC-PC-13044/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

