Application of Phosphonium Ionic Liquids as Ion Carriers in Polymer Inclusion Membranes (PIMs) for Separation of Cadmium(II) and Copper(II) from Aqueous Solutions
- PMID: 26659810
- PMCID: PMC4666278
- DOI: 10.1007/s10953-015-0413-2
Application of Phosphonium Ionic Liquids as Ion Carriers in Polymer Inclusion Membranes (PIMs) for Separation of Cadmium(II) and Copper(II) from Aqueous Solutions
Abstract
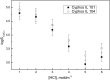
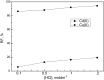
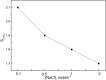
Facilitated transport through polymer inclusion membranes (PIMs) is a promising method for simultaneous separation and removal of valuable and toxic metal ions from aqueous solutions. Recently, ionic liquids (ILs) have been used as extracting agents for metal ions due to their unique physicochemical properties. This paper presents research on the facilitated transport of cadmium(II) and copper(II) ions from aqueous chloride solutions through PIMs with phosphonium ILs as new selective ion carriers. Cellulose triacetate membranes containing o-nitrophenyl octyl ether (ONPOE) as a plasticizer and Cyphos IL 101 [trihexyl(tetradecyl)phosphonium chloride] or Cyphos IL 104 [trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate] as the ion carriers have been prepared and applied for investigations. Cd(II) ions were transported preferably from hydrochloric acid solutions containing Cu(II) ions through the PIMs. Higher selectivity coefficient of Cd(II) over Cu(II) (SCd/Cu) from 0.1 mol·dm-3 hydrochloric acid was obtained for PIM with Cyphos IL 104 as the ion carrier. The influence of HCl and NaCl concentrations in the source phase on metal ion transport across PIM doped with Cyphos 104 was studied. It was found that the initial fluxes of Cd(II) and Cu(II) increase with increasing chloride ions concentration in the source phase. The selectivity coefficient for Cd(II) over Cu(II) decreases with increasing HCl concentration in the source phase. The results suggest that the separation system presented in this paper can be useful for the removal of Cd(II) from acidic chloride solutions in the presence of Cu(II).
Keywords: Cadmium(II); Copper(II); Cyphos IL 101; Cyphos IL 104; Polymer inclusion membrane (PIM).
Figures




Similar articles
-
Phosphonium ionic liquids as extractants for recovery of ruthenium(III) from acidic aqueous solutions.Chem Zvesti. 2017;71(6):1065-1072. doi: 10.1007/s11696-016-0027-1. Epub 2016 Dec 21. Chem Zvesti. 2017. PMID: 28553005 Free PMC article.
-
The Use of Polymer Membranes for the Recovery of Copper, Zinc and Nickel from Model Solutions and Jewellery Waste.Polymers (Basel). 2023 Feb 24;15(5):1149. doi: 10.3390/polym15051149. Polymers (Basel). 2023. PMID: 36904389 Free PMC article.
-
Extraction of Gold(III) from Hydrochloric Acid Solutions with a PVC-based Polymer Inclusion Membrane (PIM) Containing Cyphos(®) IL 104.Membranes (Basel). 2015 Dec 8;5(4):903-14. doi: 10.3390/membranes5040903. Membranes (Basel). 2015. PMID: 26670259 Free PMC article.
-
Application of Hydrophobic Alkylimidazoles in the Separation of Non-Ferrous Metal Ions across Plasticised Membranes-A Review.Membranes (Basel). 2020 Nov 6;10(11):331. doi: 10.3390/membranes10110331. Membranes (Basel). 2020. PMID: 33172183 Free PMC article. Review.
-
The Use of Polymer Inclusion Membranes for the Removal of Metal Ions from Aqueous Solutions-The Latest Achievements and Potential Industrial Applications: A Review.Membranes (Basel). 2022 Nov 11;12(11):1135. doi: 10.3390/membranes12111135. Membranes (Basel). 2022. PMID: 36422127 Free PMC article. Review.
Cited by
-
Polymeric Inclusion Membranes Based on Ionic Liquids for Selective Separation of Metal Ions.Membranes (Basel). 2023 Sep 13;13(9):795. doi: 10.3390/membranes13090795. Membranes (Basel). 2023. PMID: 37755217 Free PMC article.
-
Simultaneous Recovery of Precious and Heavy Metal Ions from Waste Electrical and Electronic Equipment (WEEE) Using Polymer Films Containing Cyphos IL 101.Polymers (Basel). 2021 Apr 30;13(9):1454. doi: 10.3390/polym13091454. Polymers (Basel). 2021. PMID: 33946200 Free PMC article.
-
Phosphonium ionic liquids as extractants for recovery of ruthenium(III) from acidic aqueous solutions.Chem Zvesti. 2017;71(6):1065-1072. doi: 10.1007/s11696-016-0027-1. Epub 2016 Dec 21. Chem Zvesti. 2017. PMID: 28553005 Free PMC article.
-
The Use of Polymer Membranes for the Recovery of Copper, Zinc and Nickel from Model Solutions and Jewellery Waste.Polymers (Basel). 2023 Feb 24;15(5):1149. doi: 10.3390/polym15051149. Polymers (Basel). 2023. PMID: 36904389 Free PMC article.
References
-
- Cote G. Hydrometallurgy of strategic metals. Solvent Extr. Ion Exch. 2000;18:703–727. doi: 10.1080/07366290008934704. - DOI
-
- Kolodynska D, Hubicka H, Hubicki Z. Sorption of heavy metal ions from aqueous in the presence of EDTA on monodisperse anion exchangers. Desalination. 2008;227:150–166. doi: 10.1016/j.desal.2007.06.022. - DOI
-
- Kumbasar RA. Extraction and concentration study of cadmium from zinc plant leach solutions by emulsion liquid membrane using tioctylamine as extractant. Hydrometallurgy. 2009;95:290–296. doi: 10.1016/j.hydromet.2008.07.001. - DOI
-
- De San Miguel ER, Monroy-Baretto M, Aguilar JC, Ocampo AL, Gyves J. Structural effects on metal ion migration across polymer inclusion membranes: Dependence on membrane properties and transport profiles on the weight and volume fractions of the components. J. Membr. Sci. 2011;379:416–425. doi: 10.1016/j.memsci.2011.06.013. - DOI
-
- Radzyminska-Lenarcik E, Ulewicz M. The use of 1-alkylimidzoles for selective separation of zinc ions in the transport process across a polymeric inclusion membrane. Physicochem. Probl. Miner. Process. 2014;51:131–142.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
