Phase I/II trial of weekly bortezomib with lenalidomide and dexamethasone in first relapse or primary refractory myeloma
- PMID: 26659914
- PMCID: PMC5004394
- DOI: 10.3324/haematol.2015.132431
Phase I/II trial of weekly bortezomib with lenalidomide and dexamethasone in first relapse or primary refractory myeloma
Keywords: VRD; escalated dose; first relapse; multiple myeloma.
Figures

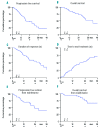
References
-
- Richardson PG, Mitsiades C, Schlossman R, Munshi N, Anderson K. New drugs for myeloma. Oncologist. 2007;12(6):664–689. - PubMed
-
- Jagannath S, Barlogie B, Berenson J, et al. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–172. - PubMed
-
- Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003; 348(26):2609–2617. - PubMed
-
- Richardson PG, Sonneveld P, Schuster MW, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903. - PubMed
-
- Ciolli S, Leoni F, Casini C, Breschi C, Santini V, Bosi A. The addition of liposomal doxorubicin to bortezomib, thalidomide and dexamethasone significantly improves clinical outcome of advanced multiple myeloma. Br J Haematol. 2008;141(6):814–819. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

