Rationale for optimal obinutuzumab/GA101 dosing regimen in B-cell non-Hodgkin lymphoma
- PMID: 26659915
- PMCID: PMC4938339
- DOI: 10.3324/haematol.2015.133421
Rationale for optimal obinutuzumab/GA101 dosing regimen in B-cell non-Hodgkin lymphoma
Abstract
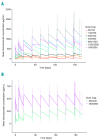
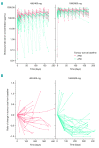
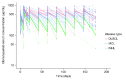
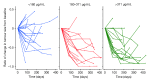
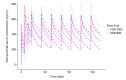
Obinutuzumab (GA101) is a type II, glycoengineered anti-CD20 monoclonal antibody for the treatment of hematologic malignancies. Obinutuzumab has mechanisms of action that are distinct from those of rituximab, potentially translating into improved clinical efficacy. We present the pharmacokinetic and clinical data from the phase I/II GAUGUIN and phase I GAUDI studies that were used to identify the obinutuzumab dose and regimen undergoing phase III assessment. In phase I (GAUGUIN and GAUDI), non-Hodgkin lymphoma patients received up to a maximum 9 fixed doses (obinutuzumab 50-2000 mg). In GAUGUIN phase II, patients received obinutuzumab 400/400 mg or 1600/800 mg [first dose day (D)1, D8, cycle (C) 1; second dose D1, C2-C8]. The influence of demographic factors on pharmacokinetics and drug exposure on tumor response and toxicity were analyzed using exploratory graphical analyses. Obinutuzumab serum concentrations with 1600/800 mg were compared with a 1000 mg fixed-dose regimen (D1, D8 and D15, C1; D1, C2-C8) using pharmacokinetic modeling simulations. Factors related to CD20-antigenic mass were more influential on obinutuzumab pharmacokinetics with 400/400 versus 1600/800 mg. Higher serum concentrations were observed with 1600/800 versus 400/400 mg, irrespective of CD20-antigenic mass. Tumor shrinkage was greater with 1600/800 versus 400/400 mg; there was no significant increase in adverse events. Fixed dose 1000 mg with an additional C1 infusion resulted in similar serum concentrations to 1600/800 mg in model-based analyses. The obinutuzumab 1000 mg fixed-dose regimen identified in this exploratory analysis was confirmed in a full covariate analysis of a larger dataset, and is undergoing phase III evaluation. GAUGUIN and GAUDI are registered at www.clinicaltrials.gov (clinicaltrials.gov identifier:00517530 and 00825149, respectively).
Trial registration: ClinicalTrials.gov NCT00517530 NCT00825149.
Copyright© Ferrata Storti Foundation.
Figures

References
-
- Coiffier B, Lepage E, Briere J, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235–242. - PubMed
-
- Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010;116(12):2040–2045. - PMC - PubMed
-
- Marcus R, Imrie K, Solal-Céligny P, et al. Phase III study of R-CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. J Clin Oncol. 2008;26(28):4579–4586. - PubMed
-
- Salles G, Mounier N, de Guibert S, et al. Rituximab combined with chemotherapy and interferon in follicular lymphoma patients: results of the GELA-GOELAMS FL2000 study. Blood. 2008;112(13):4824–4831. - PubMed
-
- Hallek M, Fischer K, Fingerle-Rowson G, et al. ; International Group of Investigators; German Chronic Lymphocytic Leukaemia Study Group. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–1174. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

