Polarity and cell division orientation in the cleavage embryo: from worm to human
- PMID: 26660321
- PMCID: PMC5062000
- DOI: 10.1093/molehr/gav068
Polarity and cell division orientation in the cleavage embryo: from worm to human
Abstract
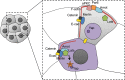
Cleavage is a period after fertilization, when a 1-cell embryo starts developing into a multicellular organism. Due to a series of mitotic divisions, the large volume of a fertilized egg is divided into numerous smaller, nucleated cells-blastomeres. Embryos of different phyla divide according to different patterns, but molecular mechanism of these early divisions remains surprisingly conserved. In the present paper, we describe how polarity cues, cytoskeleton and cell-to-cell communication interact with each other to regulate orientation of the early embryonic division planes in model animals such as Caenorhabditis elegans, Drosophila and mouse. We focus particularly on the Par pathway and the actin-driven cytoplasmic flows that accompany it. We also describe a unique interplay between Par proteins and the Hippo pathway in cleavage mammalian embryos. Moreover, we discuss the potential meaning of polarity, cytoplasmic dynamics and cell-to-cell communication as quality biomarkers of human embryos.
Keywords: C. elegans; Drosophila; Hippo signalling; cytoplasmic flow; embryo; human; mouse; par proteins; polarity; preimplantation development.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology.
Figures

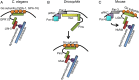

References
-
- Afshar K, Willard FS, Colombo K, Johnston CA, McCudden CR, Siderovski DP, Gönczy P. RIC-8 is required for GPR-1/2-dependent Galpha function during asymmetric division of C. elegans embryos. Cell 2004;119:219–230. - PubMed
-
- Afshar K, Willard FS, Colombo K, Siderovski DP, Gönczy P. Cortical localization of the Galpha protein GPA-16 requires RIC-8 function during C. elegans asymmetric cell division. Development 2005;132:4449–4459. - PubMed
-
- Ajduk A, Zernicka-Goetz M. Quality control of embryo development. Mol Aspects Med 2013;34:903–918. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

