BACE-1 is expressed in the blood-brain barrier endothelium and is upregulated in a murine model of Alzheimer's disease
- PMID: 26661166
- PMCID: PMC4929696
- DOI: 10.1177/0271678X15606463
BACE-1 is expressed in the blood-brain barrier endothelium and is upregulated in a murine model of Alzheimer's disease
Abstract
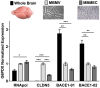
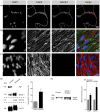
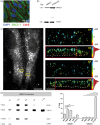
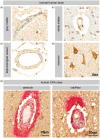
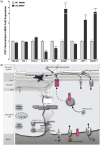
Endothelial cells of the blood-brain barrier form a structural and functional barrier maintaining brain homeostasis via paracellular tight junctions and specific transporters such as P-glycoprotein. The blood-brain barrier is responsible for negligible bioavailability of many neuroprotective drugs. In Alzheimer's disease, current treatment approaches include inhibitors of BACE-1 (β-site of amyloid precursor protein cleaving enzyme), a proteinase generating neurotoxic β-amyloid. It is known that BACE-1 is highly expressed in endosomes and membranes of neurons and glia. We now provide evidence that BACE-1 is expressed in blood-brain barrier endothelial cells of human, mouse, and bovine origin. We further show its predominant membrane localization by 3D-dSTORM super-resolution microscopy, and by biochemical fractionation that further shows an abluminal distribution of BACE-1 in brain microvessels. We confirm its functionality in processing APP in primary mouse brain endothelial cells. In an Alzheimer's disease mouse model we show that BACE-1 is upregulated at the blood-brain barrier compared to healthy controls. We therefore suggest a critical role for BACE-1 at the blood-brain barrier in β-amyloid generation and in vascular aspects of Alzheimer's disease, particularly in the development of cerebral amyloid angiopathy.
Keywords: Alzheimer’s disease; BACE-1; blood–brain barrier; endothelium; β-amyloid.
© The Author(s) 2015.
Figures

References
-
- Probst G, Xu Y-Z. Small-molecule BACE1 inhibitors: a patent literature review (2006-2011). Expert Opin Ther Pat 2012; 22: 511–540. - PubMed
-
- Bush AI, Martins RN, Rumble B, et al. The amyloid precursor protein of Alzheimer's disease is released by human platelets. J Biol Chem 1990; 265: 15977–15983. - PubMed
-
- Bush AI, Beyreuther K, Masters CL. The beta A4 amyloid protein precursor in human circulation. Ann N Y Acad Sci 1993; 695: 175–182. - PubMed
-
- Ho L, Fukuchi KI, Younkin SG. The alternatively spliced Kunitz protease inhibitor domain alters amyloid beta protein precursor processing and amyloid beta protein production in cultured cells. J Biol Chem 1996; 271: 30929–30934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

