Hemichannel-mediated release of lactate
- PMID: 26661210
- PMCID: PMC4900446
- DOI: 10.1177/0271678X15611912
Hemichannel-mediated release of lactate
Abstract
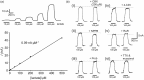
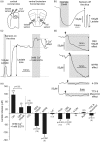
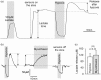
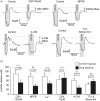
In the central nervous system lactate contributes to the extracellular pool of readily available energy substrates and may also function as a signaling molecule which mediates communication between glial cells and neurons. Monocarboxylate transporters are believed to provide the main pathway for lactate transport across the membranes. Here we tested the hypothesis that lactate could also be released via opening of pannexin and/or functional connexin hemichannels. In acute slices prepared from the brainstem, hippocampus, hypothalamus and cortex of adult rats, enzymatic amperometric biosensors detected significant tonic lactate release inhibited by compounds, which block pannexin/connexin hemichannels and facilitated by lowering extracellular [Ca(2+)] or increased PCO2 Enhanced lactate release triggered by hypoxia was reduced by ∼50% by either connexin or monocarboxylate transporter blockers. Stimulation of Schaffer collateral fibers triggered lactate release in CA1 area of the hippocampus, which was facilitated in conditions of low extracellular [Ca(2+)], markedly reduced by blockade of connexin hemichannels and abolished by lactate dehydrogenase inhibitor oxamate. These results indicate that lactate transport across the membranes may occur via mechanisms other than monocarboxylate transporters. In the central nervous system, hemichannels may function as a conduit of lactate release, and this mechanism is recruited during hypoxia and periods of enhanced neuronal activity.
Keywords: Astrocytes; connexin; lactate; metabolism; monocarboxylate transporters; pannexin.
© The Author(s) 2015.
Figures
References
-
- Ide K, Horn A, Secher N. Cerebral metabolic response to submaximal exercise. J Appl Physiol 1999; 87: 1604–1608. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

