Ion channel networks in the control of cerebral blood flow
- PMID: 26661232
- PMCID: PMC4794103
- DOI: 10.1177/0271678X15616138
Ion channel networks in the control of cerebral blood flow
Abstract
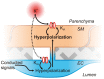
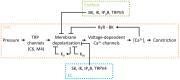
One hundred and twenty five years ago, Roy and Sherrington made the seminal observation that neuronal stimulation evokes an increase in cerebral blood flow.(1) Since this discovery, researchers have attempted to uncover how the cells of the neurovascular unit-neurons, astrocytes, vascular smooth muscle cells, vascular endothelial cells and pericytes-coordinate their activity to control this phenomenon. Recent work has revealed that ionic fluxes through a diverse array of ion channel species allow the cells of the neurovascular unit to engage in multicellular signaling processes that dictate local hemodynamics.In this review we center our discussion on two major themes: (1) the roles of ion channels in the dynamic modulation of parenchymal arteriole smooth muscle membrane potential, which is central to the control of arteriolar diameter and therefore must be harnessed to permit changes in downstream cerebral blood flow, and (2) the striking similarities in the ion channel complements employed in astrocytic endfeet and endothelial cells, enabling dual control of smooth muscle from either side of the blood-brain barrier. We conclude with a discussion of the emerging roles of pericyte and capillary endothelial cell ion channels in neurovascular coupling, which will provide fertile ground for future breakthroughs in the field.
Keywords: Ion channels; astrocytic endfoot; calcium channels; calcium signaling; cerebral blood flow; cerebrovascular resistance; endothelium; functional hyperemia; neurovascular coupling; neurovascular unit; parenchymal arteriole; pial artery; potassium channels; smooth muscle; transient receptor potential channels.
© The Author(s) 2015.
Figures






References
-
- Jennings JR, Muldoon MF, Ryan C, et al. Reduced cerebral blood flow response and compensation among patients with untreated hypertension. Neurology 2005; 64: 1358–1365. - PubMed
-
- Prunell GF, Mathiesen T, Svendgaard N-A. Experimental subarachnoid hemorrhage: cerebral blood flow and brain metabolism during the acute phase in three different models in the rat. Neurosurgery 2004; 54: 426–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

