Solution-State (17)O Quadrupole Central-Transition NMR Spectroscopy in the Active Site of Tryptophan Synthase
- PMID: 26661504
- PMCID: PMC4752203
- DOI: 10.1002/anie.201508898
Solution-State (17)O Quadrupole Central-Transition NMR Spectroscopy in the Active Site of Tryptophan Synthase
Abstract
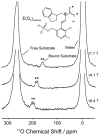
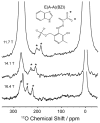
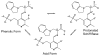
Oxygen is an essential participant in the acid-base chemistry that takes place within many enzyme active sites, yet has remained virtually silent as a probe in NMR spectroscopy. Here, we demonstrate the first use of solution-state (17)O quadrupole central-transition NMR spectroscopy to characterize enzymatic intermediates under conditions of active catalysis. In the 143 kDa pyridoxal-5'-phosphate-dependent enzyme tryptophan synthase, reactions of the α-aminoacrylate intermediate with the nucleophiles indoline and 2-aminophenol correlate with an upfield shift of the substrate carboxylate oxygen resonances. First principles calculations suggest that the increased shieldings for these quinonoid intermediates result from the net increase in the charge density of the substrate-cofactor π-bonding network, particularly at the adjacent α-carbon site.
Keywords: NMR spectroscopy; enzymes; homogeneous catalysis; ligases; proteins.
© 2016 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
Similar articles
-
Imaging active site chemistry and protonation states: NMR crystallography of the tryptophan synthase α-aminoacrylate intermediate.Proc Natl Acad Sci U S A. 2022 Jan 11;119(2):e2109235119. doi: 10.1073/pnas.2109235119. Proc Natl Acad Sci U S A. 2022. PMID: 34996869 Free PMC article.
-
X-ray and NMR crystallography in an enzyme active site: the indoline quinonoid intermediate in tryptophan synthase.J Am Chem Soc. 2011 Jan 12;133(1):4-7. doi: 10.1021/ja106555c. Epub 2010 Dec 10. J Am Chem Soc. 2011. PMID: 21142052
-
NMR crystallography of enzyme active sites: probing chemically detailed, three-dimensional structure in tryptophan synthase.Acc Chem Res. 2013 Sep 17;46(9):2008-17. doi: 10.1021/ar3003333. Epub 2013 Mar 28. Acc Chem Res. 2013. PMID: 23537227 Free PMC article.
-
Stereospecificity of alpha-proton exchange reactions catalysed by pyridoxal-5'-phosphate-dependent enzymes.Biochim Biophys Acta. 2003 Apr 11;1647(1-2):138-42. doi: 10.1016/s1570-9639(03)00080-3. Biochim Biophys Acta. 2003. PMID: 12686123 Review.
-
Channeling of substrates and intermediates in enzyme-catalyzed reactions.Annu Rev Biochem. 2001;70:149-80. doi: 10.1146/annurev.biochem.70.1.149. Annu Rev Biochem. 2001. PMID: 11395405 Review.
Cited by
-
Solid-state 17O NMR study of α-d-glucose: exploring new frontiers in isotopic labeling, sensitivity enhancement, and NMR crystallography.Chem Sci. 2022 Jan 3;13(9):2591-2603. doi: 10.1039/d1sc06060k. eCollection 2022 Mar 2. Chem Sci. 2022. PMID: 35340864 Free PMC article.
-
17 O NMR Studies of Yeast Ubiquitin in Aqueous Solution and in the Solid State.Chembiochem. 2021 Mar 2;22(5):826-829. doi: 10.1002/cbic.202000659. Epub 2020 Nov 6. Chembiochem. 2021. PMID: 33058374 Free PMC article.
-
Imaging active site chemistry and protonation states: NMR crystallography of the tryptophan synthase α-aminoacrylate intermediate.Proc Natl Acad Sci U S A. 2022 Jan 11;119(2):e2109235119. doi: 10.1073/pnas.2109235119. Proc Natl Acad Sci U S A. 2022. PMID: 34996869 Free PMC article.
-
NMR Crystallography of a Carbanionic Intermediate in Tryptophan Synthase: Chemical Structure, Tautomerization, and Reaction Specificity.J Am Chem Soc. 2016 Nov 23;138(46):15214-15226. doi: 10.1021/jacs.6b08937. Epub 2016 Nov 11. J Am Chem Soc. 2016. PMID: 27779384 Free PMC article.
-
Functional stability of water wire-carbonyl interactions in an ion channel.Proc Natl Acad Sci U S A. 2020 Jun 2;117(22):11908-11915. doi: 10.1073/pnas.2001083117. Epub 2020 May 15. Proc Natl Acad Sci U S A. 2020. PMID: 32414918 Free PMC article.
References
-
- Chan-Huot M, Dos A, Zander R, Sharif S, Tolstoy PM, Compton S, Fogle E, Toney MD, Shenderovich I, Denisov GS, Limbach HH. J Am Chem Soc. 2013;135:18160–18175. - PubMed
-
- Lai JF, Niks D, Wang YC, Domratcheva T, Barends TRM, Schwarz F, Olsen RA, Elliott DW, Fatmi MQ, Chang CEA, Schlichting I, Dunn MF, Mueller LJ. J Am Chem Soc. 2011;133:4–7. - PubMed
- Caulkins BG, Bastin B, Yang C, Neubauer TJ, Young RP, Hilario E, Huang YMM, Chang CEA, Fan L, Dunn MF, Marsella MJ, Mueller LJ. J Am Chem Soc. 2014;136:12824–12827. - PMC - PubMed
-
- Gerothanassis IP. Prog Nucl Magn Reson Spectrosc. 2010;56:95–197. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

