Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study
- PMID: 26661514
- PMCID: PMC5064615
- DOI: 10.1111/dom.12602
Once-weekly glucagon-like peptide-1 receptor agonist dulaglutide significantly decreases glycated haemoglobin compared with once-daily liraglutide in Japanese patients with type 2 diabetes: 52 weeks of treatment in a randomized phase III study
Abstract
Aims: To examine the efficacy and safety of once-weekly dulaglutide 0.75 mg monotherapy compared with once-daily liraglutide 0.9 mg in Japanese patients with type 2 diabetes (T2D) for 52 weeks.
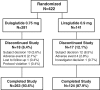
Methods: We conducted a phase III, randomized, 52-week (26-week primary endpoint), active- and placebo-controlled trial comparing 492 Japanese patients (dulaglutide, n = 281; liraglutide, n = 141; and placebo, n = 70). Participants and investigators were blinded to treatment assignment for dulaglutide and placebo but not for liraglutide (open-label comparator); after 26 weeks, patients randomized to placebo were switched to once-weekly dulaglutide 0.75 mg (open-label). The present paper reports results for patients treated with dulaglutide and patients treated with liraglutide for 52 weeks.
Results: At week 52, dulaglutide decreased HbA1c significantly from baseline compared with liraglutide [least squares mean difference: -0.20; 95% confidence interval (CI) -0.39, -0.01; p = 0.04]. At week 52 (last observation carried forward), dulaglutide significantly decreased pre- and post-dinner blood glucose (BG) levels, the mean of seven-point self-monitored BG profiles, the mean of all postprandial BG levels and circadian variation compared with liraglutide. Body weight was generally stable in both groups through 52 weeks. The most frequently reported adverse events were nasopharyngitis, constipation, nausea and diarrhoea. Eight dulaglutide-treated (2.9%) and four liraglutide-treated (2.9%) patients reported hypoglycaemia, with no event being severe.
Conclusions: Monotherapy with once-weekly dulaglutide 0.75 mg was effective and safe in Japanese patients with T2D, with better glycaemic control compared with once-daily liraglutide 0.9 mg.
Keywords: GLP-1 receptor agonist; dulaglutide; liraglutide; type 2 diabetes.
© 2015 The Authors. Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Figures


References
-
- Byetta ([exenatide] injection) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available from URL: http://www.azpicentral.com/byetta/pi_byetta.pdf. Accessed 10 July 2015.
-
- Sanofi‐Aventis Deutschland GmbH Lixisenatide (Lyxumia) . Summary of product characteristics. EMA website. Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_‐_Product_Info.... Accessed 10 July 2015.
-
- Victoza® (liraglutide [rDNA origin] injection, solution for subcutaneous use) [prescribing information]. Bagsvaerd, Denmark: Novo Nordisk A/S; 2015. Available from URL: http://www.novo‐pi.com/victoza.pdf. Accessed 10 July 2015.
-
- Bydureon® ([exenatide extended‐release] for injectable suspension) [prescribing information]. Wilmington, DE: AstraZeneca Pharmaceuticals LP; 2015. Available from URL: http://www.azpicentral.com/bydureon/pi_bydureon.pdf Accessed 10 July 2015.
-
- Tanzeum ([albiglutide] for injection, for subcutaneous use) [prescribing information]. Wilmington, DE: GlaxoSmithKline LLC; 2015. Available from URL: https://gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribi.... Accessed 10 July 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

