Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells
- PMID: 26661657
- PMCID: PMC4678765
- DOI: 10.1371/journal.pone.0145068
Collagen Promotes Higher Adhesion, Survival and Proliferation of Mesenchymal Stem Cells
Abstract
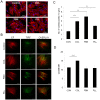
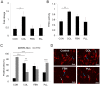
Mesenchymal stem cells (MSC) can differentiate into several cell types and are desirable candidates for cell therapy and tissue engineering. However, due to poor cell survival, proliferation and differentiation in the patient, the therapy outcomes have not been satisfactory. Although several studies have been done to understand the conditions that promote proliferation, differentiation and migration of MSC in vitro and in vivo, still there is no clear understanding on the effect of non-cellular bio molecules. Of the many factors that influence the cell behavior, the immediate cell microenvironment plays a major role. In this context, we studied the effect of extracellular matrix (ECM) proteins in controlling cell survival, proliferation, migration and directed MSC differentiation. We found that collagen promoted cell proliferation, cell survival under stress and promoted high cell adhesion to the cell culture surface. Increased osteogenic differentiation accompanied by high active RHOA (Ras homology gene family member A) levels was exhibited by MSC cultured on collagen. In conclusion, our study shows that collagen will be a suitable matrix for large scale production of MSC with high survival rate and to obtain high osteogenic differentiation for therapy.
Conflict of interest statement
Figures



References
-
- Toma C, Pittenger MF, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8. Epub 2002/01/05. . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

