The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent
- PMID: 26662461
- PMCID: PMC5066736
- DOI: 10.1002/hipo.22555
The requirement of BDNF for hippocampal synaptic plasticity is experience-dependent
Abstract
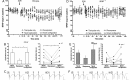
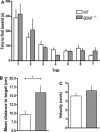
Brain-derived neurotrophic factor (BDNF) supports neuronal survival, growth, and differentiation and has been implicated in forms of hippocampus-dependent learning. In vitro, a specific role in hippocampal synaptic plasticity has been described, although not all experience-dependent forms of synaptic plasticity critically depend on BDNF. Synaptic plasticity is likely to enable long-term synaptic information storage and memory, and the induction of persistent (>24 h) forms, such as long-term potentiation (LTP) and long-term depression (LTD) is tightly associated with learning specific aspects of a spatial representation. Whether BDNF is required for persistent (>24 h) forms of LTP and LTD, and how it contributes to synaptic plasticity in the freely behaving rodent has never been explored. We examined LTP, LTD, and related forms of learning in the CA1 region of freely dependent mice that have a partial knockdown of BDNF (BDNF(+/-) ). We show that whereas early-LTD (<90min) requires BDNF, short-term depression (<45 min) does not. Furthermore, BDNF is required for LTP that is induced by mild, but not strong short afferent stimulation protocols. Object-place learning triggers LTD in the CA1 region of mice. We observed that object-place memory was impaired and the object-place exploration failed to induce LTD in BDNF(+/-) mice. Furthermore, spatial reference memory, that is believed to be enabled by LTP, was also impaired. Taken together, these data indicate that BDNF is required for specific, but not all, forms of hippocampal-dependent information storage and memory. Thus, very robust forms of synaptic plasticity may circumvent the need for BDNF, rather it may play a specific role in the optimization of weaker forms of plasticity. The finding that both learning-facilitated LTD and spatial reference memory are both impaired in BDNF(+/-) mice, suggests moreover, that it is critically required for the physiological encoding of hippocampus-dependent memory. © 2015 The Authors Hippocampus Published by Wiley Periodicals, Inc.
Keywords: CA1; LTD; LTP; enrichment; object recognition.
© 2015 Wiley Periodicals, Inc.
Figures


References
-
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens CF, Tonegawa S. 1994. Reduced hippocampal long‐term potentiation and context‐specific deficit in associative learning in mGluR1 mutant mice. Cell 79:365–375. - PubMed
-
- Bevins RA, Besheer J. 2006. Object recognition in rats and mice: A one‐trial non‐matching‐to‐sample learning task to study 'recognition memory’. Nat Protoc 1:1306–1311. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

