Ascorbic acid for the treatment of Charcot-Marie-Tooth disease
- PMID: 26662471
- PMCID: PMC6823270
- DOI: 10.1002/14651858.CD011952
Ascorbic acid for the treatment of Charcot-Marie-Tooth disease
Abstract
Background: Charcot-Marie-Tooth disease (CMT) comprises a large group of different forms of hereditary motor and sensory neuropathy. The molecular basis of several CMT subtypes has been clarified during the last 20 years. Since slowly progressive muscle weakness and sensory disturbances are the main features of these syndromes, treatments aim to improve motor impairment and sensory disturbances to improve abilities. Pharmacological treatment trials in CMT are rare. This review was derived from a Cochrane review, Treatment for Charcot Marie Tooth disease, which will be updated via this review and a forthcoming title, Treatments other than ascorbic acid for Charcot-Marie-Tooth disease.
Objectives: To assess the effects of ascorbic acid (vitamin C) treatment for CMT.
Search methods: On 21 September 2015, we searched the Cochrane Neuromuscular Specialised Register, Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and LILACS for randomised controlled trials (RCTs) of treatment for CMT. We also checked clinical trials registries for ongoing studies.
Selection criteria: We included RCTs and quasi-RCTs of any ascorbic acid treatment for people with CMT. Where a study aimed to evaluate the treatment of general neuromuscular symptoms of people with peripheral neuropathy including CMT, we included the study if we were able to identify the effect of treatment in the CMT group. We did not include observational studies or case reports of ascorbic acid treatment in people with CMT.
Data collection and analysis: Two review authors (BG and JB) independently extracted the data and assessed study quality.
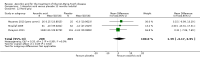
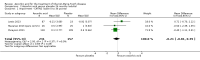
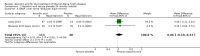
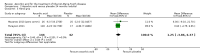
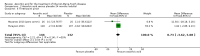
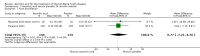
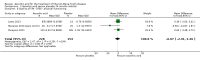
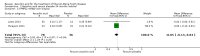
Main results: Six RCTs compared the effect of oral ascorbic acid (1 to 4 grams) and placebo treatment in CMT1A. In five trials involving adults with CMT1A, a total of 622 participants received ascorbic acid or placebo. Trials were largely at low risk of bias. There is high-quality evidence that ascorbic acid does not improve the course of CMT1A in adults as measured by the CMT neuropathy score (0 to 36 scale) at 12 months (mean difference (MD) -0.37; 95% confidence intervals (CI) -0.83 to 0.09; five studies; N = 533), or at 24 months (MD -0.21; 95% CI -0.81 to 0.39; three studies; N = 388). Ascorbic acid treatment showed a positive effect on the nine-hole peg test versus placebo (MD -1.16 seconds; 95% CI -1.96 to -0.37), but the clinical significance of this result is probably small. Meta-analyses of other secondary outcome parameters showed no relevant benefit of ascorbic acid. In one trial, 80 children with CMT1A received ascorbic acid or placebo. The trial showed no clinical benefit of ascorbic acid treatment. Adverse effects did not differ in their nature or abundance between ascorbic acid and placebo.
Authors' conclusions: High-quality evidence indicates that ascorbic acid does not improve the course of CMT1A in adults in terms of the outcome parameters used. According to low-quality evidence, ascorbic acid does not improve the course of CMT1A in children. However, CMT1A is slowly progressive and the outcome parameters show only small change over time. Longer study durations should be considered, and outcome parameters more sensitive to change over time should be designed and validated for future studies.
Conflict of interest statement
MM Reilly and D Pareyson were involved as investigators in one of the trials included in this review (Pareyson 2011). B Gess and P Young have been involved in basic research projects on the function of ascorbic acid in the peripheral nervous system (Gess 2010; Gess 2011).
Figures

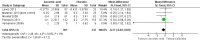

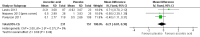













































References
References to studies included in this review
Burns 2009 {published data only}
-
- Burns J, Ouvrier RA, Yiu EM, Joseph PD, Kornberg AJ, Fahey MC, et al. Ascorbic acid for Charcot–Marie–Tooth disease type 1A in children: a randomised, double‐blind, placebo‐controlled, safety and efficacy trial. Lancet Neurology 2009;8(6):537‐44. [PUBMED: 19427269] - PubMed
Lewis 2013 {published data only}
-
- Lewis RA, McDermott MP, Herrmann DN, Hoke A, Clawson LL, Siskind C, et al. for the Muscle Study Group. High‐dosage ascorbic acid treatment in Charcot‐Marie‐Tooth disease type 1A: results of a randomized, double‐masked, controlled trial. JAMA Neurology 2013;70(8):981‐7. [PUBMED: 23797954] - PMC - PubMed
Mazanec 2013 [pers comm] {unpublished data only}
-
- Mazanec R. CMT Cochrane Review [personal communication]. Email to: B Gess 12 August 2013.
Micallef 2009 {published data only}
-
- Micallef J, Attarian S, Dubourg O, Gonnaud PM, Hogrel JY, Stojkovic T, et al. Effect of ascorbic acid in patients with Charcot‐Marie‐Tooth disease type 1A: a multicentre, randomised, double‐blind, placebo‐controlled trial. Lancet Neurology 2009;8(12):1103‐10. [PUBMED: 19818690] - PubMed
Pareyson 2011 {published data only}
Verhamme 2009a {published data only}
-
- Verhamme C, Haan RJ, Vermeulen M, Baas F, Visser M, Schaik IN. Oral high dose ascorbic acid treatment for one year in young CMT1A patients: a randomised, double‐blind, placebo‐controlled phase II trial. BMC Medicine 2009;7:70. [http://www.biomedcentral.com/1741‐7015/7/70; PUBMED: 19909499] - PMC - PubMed
References to studies excluded from this review
Toth 2009 {published data only}
-
- Toth C. Poor tolerability of high dose ascorbic acid in a population of genetically confirmed adult Charcot‐Marie‐Tooth 1A patients. Acta Neurologica Scandinavica 2009;120(2):134‐8. - PubMed
Additional references
Anderson 1996
-
- Anderson RT, Aaronson NK, Bullinger M, McBee WL. A review of the progress towards developing health‐related quality‐of‐life instruments for international clinical studies and outcomes research. Pharmacoeconomics 1996;10(4):336‐55. - PubMed
Auer‐Grumbach 2013
-
- Auer‐Grumbach M. Hereditary sensory and autonomic neuropathies. Handbook of Clinical Neurology 2013;115:893‐906. - PubMed
Baets 2014
-
- Baets J, Jonghe P, Timmerman V. Recent advances in Charcot‐Marie‐Tooth disease. Current Opinion in Neurology 2014;27(5):532‐40. - PubMed
Baxter 2002
-
- Baxter RV, Ben Othmane K, Rochelle JM, Stajich JE, Hulette C, Dew‐Knight S, et al. Ganglioside‐induced differentiation‐associated protein‐1 is mutant in Charcot‐Marie‐Tooth disease type 4A/8q21. Nature Genetics 2002;30(1):21‐2. - PubMed
Bird 2014
-
- Bird TD. Charcot‐Marie‐Tooth Hereditary Neuropathy Overview. In: Pagon RA, Adam MP, Ardinger HH, Wallace SE, Amemiya A, Bean LJH, et al. editor(s). GeneReviews [Internet]. Seattle: University of Washington, 1998 (updated 2014). - PubMed
Braathen 2012
-
- Braathen GJ. Genetic epidemiology of Charcot‐Marie‐Tooth disease. Acta Neurologica Scandinavica Supplement 2012;193(iv):22. - PubMed
Burns 2012
Chance 1993
-
- Chance PF, Alderson MK, Leppig KA, Lensch MW, Matsunami N, Smith B, et al. DNA deletion associated with hereditary neuropathy with liability to pressure palsies. Cell 1993;72(1):143‐51. - PubMed
Cornblath 1999
-
- Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, et al. Total neuropathy score: validation and reliability study. Neurology 1999;53(8):1660‐4. - PubMed
d'Ydewalle 2012
-
- d'Ydewalle C, Benoy V, Bosch L. Charcot‐Marie‐Tooth disease: emerging mechanisms and therapies. International Journal of Biochemistry and Cell Biology 2012;44(8):1299‐304. - PubMed
Dyck 2005b
-
- Dyck PJ, Hughes RAC, O'Brien PC. Quantitating overall neuropathic symptoms, impairments, and outcomes. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. 4th Edition. Philadelphia: WB Saunders, 2005:1031‐52.
Eldridge 1987
Gabreels‐Festen 1993
-
- Gabreels‐Festen A, Gabreels F. Hereditary demyelinating motor and sensory neuropathy. Brain Pathology 1993;3(2):135‐46. - PubMed
Gess 2010
-
- Gess B, Lohmann C, Halfter H, Young P. Sodium‐dependent vitamin C transporter 2 (SVCT2) is necessary for the uptake of L‐ascorbic acid into Schwann cells. Glia 2010;58(3):287‐99. - PubMed
Gess 2011
Gess 2013
-
- Gess B, Schirmacher A, Boentert M, Young P. Charcot‐Marie‐Tooth disease: frequency of genetic subtypes in a German neuromuscular center population. Neuromuscular Disorders 2013;23(8):647‐51. - PubMed
GRADEpro 2015 [Computer program]
-
- McMaster University. GRADEpro GDT: GRADEpro Guideline Development Tool [Software].(developed by Evidence Prime, Inc.). Available from gradepro.org. Computer program on www.gradepro.org]. McMaster University, 2015.
Grandis 2005
-
- Grandis M, Shy ME. Current therapy for Charcot‐Marie‐Tooth disease. Current Treatment Options in Neurology 2005;7(1):23‐31. - PubMed
Hahn 2001
-
- Hahn AF, Ainsworth PJ, Bolton CF, Bilbao JM, Vallat JM. Pathological findings in the x‐linked form of Charcot‐Marie‐Tooth disease: a morphometric and ultrastructural analysis. Acta Neuropathologica (Berlin) 2001;101(2):129‐39. - PubMed
Harding 1980
-
- Harding AE, Thomas PK. The clinical features of hereditary motor and sensory neuropathy types I and II. Brain 1980;103(2):259‐80. - PubMed
Hayasaka 1993a
-
- Hayasaka K, Himoro M, Takada G, Takahashi E, Minoshima S, Shimizu N. Structure and localization of the gene encoding human peripheral myelin protein 2 (PMP2). Genomics 1993;18(2):244‐8. - PubMed
Hayasaka 1993b
-
- Hayasaka K, Himoro M, Sawaishi Y, Nanao K, Takahashi T, Takada G, et al. De novo mutation of the myelin P0 gene in Dejerine‐Sottas disease (hereditary motor and sensory neuropathy type III). Nature Genetics 1993;5(3):266‐8. - PubMed
Hayasaka 1993c
-
- Hayasaka K, Ohnishi A, Takada G, Fukushima Y, Murai Y. Mutation of the myelin P0 gene in Charcot‐Marie‐tooth neuropathy type 1. Biochemical and Biophysical Research Communications 1993;194(3):1317‐22. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org 2011.
Jenkinson 1993
Komyathy 2013
Krajewski 1999
-
- Krajewski K, Turansky C, Lewis R, Garbern J, Hinderer S, Kamholz J, et al. Correlation between weakness and axonal loss in patients with CMT1A. Annals of the New York Academy of Science 1999;883:490‐2. - PubMed
Krajewski 2000
-
- Krajewski KM, Lewis RA, Fuerst DR, Turansky C, Hinderer SR, Garbern J, et al. Neurological dysfunction and axonal degeneration in Charcot‐Marie‐Tooth disease type 1A. Brain 2000;123(Pt 7):1516‐27. - PubMed
Kurihara 2002
-
- Kurihara S, Adachi Y, Wada K, Awaki E, Harada H, Nakashima K. An epidemiological genetic study of Charcot‐Marie‐Tooth disease in Western Japan. Neuroepidemiology 2002;21(5):246‐50. - PubMed
Liu 2014
Lupski 1991
-
- Lupski JR, Oca‐Luna RM, Slaugenhaupt S, Pentao L, Guzzetta V, Trask BJ, et al. DNA duplication associated with Charcot‐Marie‐Tooth disease type 1A. Cell 1991;66(2):219‐32. - PubMed
Mannil 2014
-
- Mannil M, Solari A, Leha A, Pelayo‐Negro AL, Berciano J, Schlotter‐Weigel B, et al. Selected items from the Charcot‐Marie‐Tooth (CMT) Neuropathy Score and secondary clinical outcome measures serve as sensitive clinical markers of disease severity in CMT1A patients. Neuromuscular Disorders 2014;24(11):1003‐17. - PubMed
Martini 2001
-
- Martini R. The effect of myelinating Schwann cells on axons. Muscle & Nerve 2001;24(4):456‐66. - PubMed
Merkies 2000
-
- Merkies IS, Schmitz PI, Meche FG, Doorn PA. Psychometric evaluation of a new sensory scale in immune‐mediated polyneuropathies. Inflammatory Neuropathy Cause and Treatment (INCAT) Group. Neurology 2000;54(4):943‐9. - PubMed
Murphy 2011
Murphy 2012
Nelis 1996
-
- Nelis E, Broeckhoven C, Jonghe P, Lofgren A, Vandenberghe A, Latour P, et al. Estimation of the mutation frequencies in Charcot‐Marie‐Tooth disease type 1 and hereditary neuropathy with liability to pressure palsies: a European collaborative study. European Journal of Human Genetics 1996;4(1):25‐33. - PubMed
Nicholson 1993
-
- Nicholson G, Nash J. Intermediate nerve conduction velocities define X‐linked Charcot‐Marie‐Tooth neuropathy families. Neurology 1993;43(12):2558‐64. - PubMed
Nobbio 2014
-
- Nobbio L, Visigalli D, Radice D, Fiorina E, Solari A, Lauria G, et al. PMP22 messenger RNA levels in skin biopsies: testing the effectiveness of a Charcot‐Marie‐Tooth 1A biomarker. Brain 2014;127(6):1614‐20. - PubMed
Pareyson 2014
-
- Pareyson D, Saveri P, Piscosquito G. Charcot‐Marie‐Tooth disease and related hereditary neuropathies: from gene function to associated phenotypes. Current Molecular Medicine 2014; Vol. Epub ahead of print. - PubMed
Passage 2004
-
- Passage E, Norreel JC, Noack‐Fraissignes P, Sanguedolce V, Pizant J, Thirion X, et al. Ascorbic acid treatment corrects the phenotype of a mouse model of Charcot‐Marie‐Tooth disease. Nature Medicine 2004;10(4):396‐401. - PubMed
Raeymakers 1991
-
- Raeymaekers P, Timmerman V, Nelis E, Jonghe P, Hoogendijk JE, Baas F, et al. Duplication in chromosome 17p11.2 in Charcot‐Marie‐Tooth neuropathy type 1a (CMT 1a). The HMSN Collaborative Research Group. Neuromuscular Disorders 1991;1(2):93‐7. - PubMed
Reilly 2006
-
- Reilly MM, Jonghe P, Pareyson D. 136th ENMC International Workshop: Charcot‐Marie‐Tooth disease type 1A (CMT1A)8‐10 April 2005, Naarden, The Netherlands. Neuromuscular Disorders 2006;16(6):396‐402. - PubMed
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Center, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014.
Rossor 2012
-
- Rossor AM, Kalmar B, Greensmith L, Reilly MM. The distal hereditary motor neuropathies. Journal of Neurology, Neurosurgery & Psychiatry 2012;83(1):6‐14. - PubMed
Rossor 2013
-
- Rossor AM, Polke JM, Houlden H, Reilly MM. Clinical implications of genetic advances in Charcot‐Marie‐Tooth disease. Nature Reviews Neurology 2013;9(10):562‐71. - PubMed
Saporta 2011
Senderek 2003
Shy 2005a
-
- Shy ME, Blake J, Krajewski K, Fuerst DR, Laura M, Hahn AF, et al. Reliability and validity of the CMT neuropathy score as a measure of disability. Neurology 2005;64(7):1209‐14. - PubMed
Shy 2005b
-
- Shy ME, Lupski JR, Chance PF, Klein CJ, Dyck PJ. Hereditary motor and sensory neuropathy. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. Vol. 2, Philadelphia: WB Saunders, 2005:1623‐58.
Timmerman 1992
-
- Timmerman V, Nelis E, Hul W, Nieuwenhuijsen B, Chen K, Wang S, et al. The peripheral myelin protein gene PMP‐22 is contained within the Charcot‐Marie‐Tooth disease type 1A duplication. Nature Genetics 1992;1(3):171‐5. - PubMed
Vallat 2013
-
- Vallat JM, Mathis S, Funalot B. The various Charcot‐Marie‐Tooth diseases. Current Opinion in Neurology 2013;26(5):473‐80. - PubMed
van Swieten 1988
-
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604‐7. - PubMed
Verhamme 2009b
-
- Verhamme C, Schaik IN, Koelman JH, Haan RJ, Visser M. The natural history of Charcot‐Marie‐Tooth type 1A in adults: a 5‐year follow‐up study. Brain 2009;132(Pt 12):3252‐62. - PubMed
Verhoeven 2006
-
- Verhoeven K, Claeys KG, Zuchner S, Schroder JM, Weis J, Ceuterick C, et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot‐Marie‐Tooth type 2. Brain 2006;129(Pt 8):2093‐102. - PubMed
Young 2001
-
- Young P, Suter U. Disease mechanisms and potential therapeutic strategies in Charcot‐Marie‐Tooth disease. Brain Research. Brain Research Reviews 2001;36(2‐3):213‐21. - PubMed
Zimon 2012
-
- Zimoń M, Baets J, Almeida‐Souza L, Vriendt E, Nikodinovic J, Parman Y, et al. Loss‐of‐function mutations in HINT1 cause axonal neuropathy with neuromyotonia. Nature Genetics 2012;44(10):1080‐3. - PubMed
Zuchner 2004
-
- Zuchner S, Mersiyanova IV, Muglia M, Bissar‐Tadmouri N, Rochelle J, Dadali EL, et al. Mutations in the mitochondrial GTPase mitofusin 2 cause Charcot‐Marie‐Tooth neuropathy type 2A. Nature Genetics 2004;36(5):449‐51. - PubMed
References to other published versions of this review
Young 2006
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

