Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons
- PMID: 26662922
- PMCID: PMC4867135
- DOI: 10.1002/hipo.22557
Activation of local inhibitory circuits in the dentate gyrus by adult-born neurons
Abstract
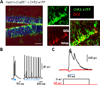
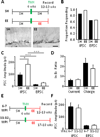
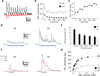
Robust incorporation of new principal cells into pre-existing circuitry in the adult mammalian brain is unique to the hippocampal dentate gyrus (DG). We asked if adult-born granule cells (GCs) might act to regulate processing within the DG by modulating the substantially more abundant mature GCs. Optogenetic stimulation of a cohort of young adult-born GCs (0 to 7 weeks post-mitosis) revealed that these cells activate local GABAergic interneurons to evoke strong inhibitory input to mature GCs. Natural manipulation of neurogenesis by aging-to decrease it-and housing in an enriched environment-to increase it-strongly affected the levels of inhibition. We also demonstrated that elevating activity in adult-born GCs in awake behaving animals reduced the overall number of mature GCs activated by exploration. These data suggest that inhibitory modulation of mature GCs may be an important function of adult-born hippocampal neurons. © 2015 Wiley Periodicals, Inc.
Keywords: adult neurogenesis; dentate gyrus; granule cells; hippocampus; inhibition; interneurons; optogenetics.
© 2015 Wiley Periodicals, Inc.
Figures
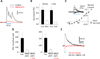


References
-
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. - PubMed
-
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. Eur J Neurosci. 2005;21:513–521. - PubMed
-
- Buhl EH, Szilágyi T, Halasy K, Somogyi P. Physiological properties of anatomically identified basket and bistratified cells in the CA1 area of the rat hippocampus in vitro. Hippocampus. 1996;6:294–305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

