Brief Report: Utilization of the First Biosimilar Infliximab Since Its Approval in South Korea
- PMID: 26662931
- PMCID: PMC4848142
- DOI: 10.1002/art.39546
Brief Report: Utilization of the First Biosimilar Infliximab Since Its Approval in South Korea
Abstract
Objective: The US Food and Drug Administration is considering an application for a biosimilar version of infliximab, which has been available in South Korea since November 2012. The aim of the present study was to examine the utilization patterns of both branded and biosimilar infliximab and other tumor necrosis factor (TNF) inhibitors in South Korea before and after the introduction of this biosimilar infliximab.
Methods: Using claims data from April 2009 to March 2014 from the Korean Health Insurance Review and Assessment Service database, which includes the entire South Korean population, the number of claims for biosimilar infliximab was assessed. A segmented linear regression model was used to examine the utilization patterns of infliximab (the branded and biosimilar versions) and other TNF inhibitors (adalimumab and etanercept) before and after the introduction of the biosimilar infliximab.
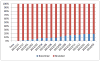
Results: In total, 20,976 TNF inhibitor users were identified from the South Korean claims database, including 983 with a prescription claim for biosimilar infliximab. Among all of the claims for any version of infliximab, the proportion of biosimilar infliximab claims increased to 19% through March 2014. Before November 2012, each month there were 33 (95% confidence interval [95% CI] 32, 35) more infliximab claims, 44 (95% CI 40, 48) more etanercept claims, and 50 (95% CI 47, 53) more adalimumab claims. After November 2012, there were significant changes in the slopes for trend in usage, with additional increases in the use of branded and biosimilar infliximab (9 more claims per month, 95% CI 2, 17) and decreases in the use of etanercept (-52 claims per month, 95% CI -66, -38) and adalimumab (-21 claims per month, 95% CI -35, -6).
Conclusion: During the first 15 months since its introduction in South Korea, one-fifth of all infliximab claims were for the biosimilar version. Introduction of biosimilar infliximab may affect the use of other TNF inhibitors, and the magnitude of change in usage will likely differ in other countries.
© 2016, American College of Rheumatology.
Conflict of interest statement
SCK receives salary support from unrelated grants to Brigham and Women’s Hospital from Pfizer, AstraZeneca and Lilly. DHS receives salary support from unrelated grants to Brigham and Women’s Hospital from Lilly, Pfizer, Amgen, and AstraZeneca. Other authors have nothing to disclose.
Figures

Comment in
-
Editorial: Biosimilars: New or Déjà Vu?Arthritis Rheumatol. 2016 May;68(5):1049-52. doi: 10.1002/art.39568. Arthritis Rheumatol. 2016. PMID: 26748580 No abstract available.
References
-
- Yoo DH. The rise of biosimilars: potential benefits and drawbacks in rheumatoid arthritis. Expert Rev Clin Immunol. 2014;10(8):981–3. - PubMed
-
- Yoo DH, Hrycaj P, Miranda P, Ramiterre E, Piotrowski M, Shevchuk S, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–20. - PMC - PubMed
-
- Park W, Hrycaj P, Jeka S, Kovalenko V, Lysenko G, Miranda P, et al. A randomised, double-blind, multicentre, parallel-group, prospective study comparing the pharmacokinetics, safety, and efficacy of CT-P13 and innovator infliximab in patients with ankylosing spondylitis: the PLANETAS study. Ann Rheum Dis. 2013;72(10):1605–12. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

