Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells
- PMID: 26663081
- PMCID: PMC4751403
- DOI: 10.1074/jbc.M115.706523
Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells
Abstract
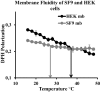
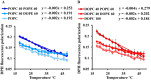
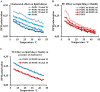
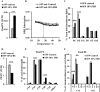
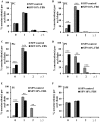
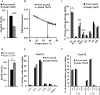
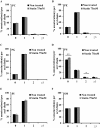
Adequate membrane fluidity is required for a variety of key cellular processes and in particular for proper function of membrane proteins. In most eukaryotic cells, membrane fluidity is known to be regulated by fatty acid desaturation and cholesterol, although some cells, such as insect cells, are almost devoid of sterol synthesis. We show here that insect and mammalian cells present similar microviscosity at their respective physiological temperature. To investigate how both sterols and phospholipids control fluidity homeostasis, we quantified the lipidic composition of insect SF9 and mammalian HEK 293T cells under normal or sterol-modified condition. As expected, insect cells show minimal sterols compared with mammalian cells. A major difference is also observed in phospholipid content as the ratio of phosphatidylethanolamine (PE) to phosphatidylcholine (PC) is inverted (4 times higher in SF9 cells). In vitro studies in liposomes confirm that both cholesterol and PE can increase rigidity of the bilayer, suggesting that both can be used by cells to maintain membrane fluidity. We then show that exogenously increasing the cholesterol amount in SF9 membranes leads to a significant decrease in PE:PC ratio whereas decreasing cholesterol in HEK 293T cells using statin treatment leads to an increase in the PE:PC ratio. In all cases, the membrane fluidity is maintained, indicating that both cell types combine regulation by sterols and phospholipids to control proper membrane fluidity.
Keywords: cholesterol; homeostasis; membrane bilayer; membrane lipid; phosphatidylethanolamine.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Nicolson G. L. (2014) The fluid-mosaic model of membrane structure: still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta 1838, 1451–1466 - PubMed
-
- Lipowsky R. (2014) Remodeling of membrane compartments: some consequences of membrane fluidity. Biol. Chem. 395, 253–274 - PubMed
-
- Cybulski L. E., del Solar G., Craig P. O., Espinosa M., and de Mendoza D. (2004) Bacillus subtilis DesR functions as a phosphorylation-activated switch to control membrane lipid fluidity. J. Biol. Chem. 279, 39340–39347 - PubMed
-
- Holthuis J. C., and Menon A. K. (2014) Lipid landscapes and pipelines in membrane homeostasis. Nature 510, 48–57 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

