Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation
- PMID: 26663221
- PMCID: PMC4755440
- DOI: 10.1093/humupd/dmv055
Extracellular vesicles: roles in gamete maturation, fertilization and embryo implantation
Abstract
Background: Extracellular vesicles (EVs) are membrane-bound vesicles, found in biofluids, that carry and transfer regulatory molecules, such as microRNAs (miRNAs) and proteins, and may mediate intercellular communication between cells and tissues. EVs have been isolated from a wide variety of biofluids, including plasma, urine, and, relevant to this review, seminal, follicular and uterine luminal fluid. We conducted a systematic search of the literature to review and present the currently available evidence on the possible roles of EVs in follicular growth, resumption of oocyte development and maturation (meiosis), sperm maturation, fertilization and embryo implantation.
Methods: MEDLINE, Embase and Web of Science databases were searched using keywords pertaining to EVs, including 'extracellular vesicles', 'microvesicles', 'microparticles' and 'exosomes', combined with a range of terms associated with the period of development between fertilization and implantation, including 'oocyte', 'sperm', 'semen', 'fertilization', 'implantation', 'embryo', 'follicular fluid', 'epididymal fluid' and 'seminal fluid'. Relevant research articles published in English (both animal and human studies) were reviewed with no restrictions on publication date (i.e. from earliest database dates to July 2015). References from these articles were used to obtain additional articles.
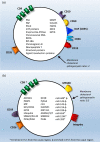
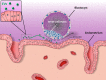
Results: A total of 1556 records were retrieved from the three databases. After removing duplicates and irrelevant titles, we reviewed the abstracts of 201 articles, which included 92 relevant articles. Both animal and human studies unequivocally identified various types of EVs in seminal, follicular and ULFs. Several studies provided evidence for the roles of EVs in these biofluids. In men, EVs in seminal fluid were linked with post-testicular sperm maturation, including sperm motility acquisition and reduction of oxidative stress. In women, EVs in follicular fluid were shown to contain miRNAs with potential roles in follicular growth, resumption of oocyte meiosis, steroidogenesis and prevention of polyspermy after fertilization. EVs were also detected in the media of cultured embryos, suggesting that EVs released from embryos and the uterus may mediate embryo-endometrium cross-talk during implantation. It is important to note that many of the biologically plausible functions of EVs in reproduction discussed in the current literature have not yet been substantiated by conclusive experimental evidence.
Conclusions: A detailed understanding of the contributions of EVs in the series of events from gametogenesis to fertilization and then on to implantation, in both normal and pathological cases, may enable the development of valuable tools to advance reproductive health. Because of the early stage of the field, it is unsurprising that the current literature includes not only growing experimental evidence, but also as-yet unproven hypotheses pertaining to the roles of EVs in key reproductive processes. In this review, we present a comprehensive survey of the rapidly expanding literature on this subject, highlighting both relevant findings and gaps in knowledge.
Keywords: embryo; exosomes; extracellular vesicles; fertilization; implantation; oocyte; ovarian follicle; sperm.
© The Author 2015. Published by Oxford University Press on behalf of the European Society of Human Reproduction and Embryology. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Aalberts M, Sostaric E, Wubbolts R, Wauben MWM, Nolte THENM, Gadella BM, Stout TAE, Stoorvogel W. Spermatozoa recruit prostasomes in response to capacitation induction. Biochim Biophys Acta 2013;1834:2326–2335. - PubMed
-
- Adashi EY. Endocrinology of the ovary. Hum Reprod 1994;9:815–827. - PubMed
-
- Aitken RJ, Nixon B. Sperm capacitation: a distant landscape glimpsed but unexplored. Mol Hum Reprod 2013;19:785–793. - PubMed
-
- Arienti G, Carlini E, Palmerini CA. Fusion of human sperm to prostasomes at acidic pH. J Membr Biol 1997;155:89–94. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

