Endothelin regulates intermittent hypoxia-induced lipolytic remodelling of adipose tissue and phosphorylation of hormone-sensitive lipase
- PMID: 26663321
- PMCID: PMC4799974
- DOI: 10.1113/JP271321
Endothelin regulates intermittent hypoxia-induced lipolytic remodelling of adipose tissue and phosphorylation of hormone-sensitive lipase
Abstract
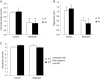
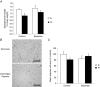
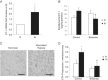
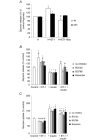
Obstructive sleep apnoea syndrome is characterized by repetitive episodes of upper airway collapse during sleep resulting in chronic intermittent hypoxia (IH). Obstructive sleep apnoea syndrome, through IH, promotes cardiovascular and metabolic disorders. Endothelin-1 (ET-1) secretion is upregulated by IH, and is able to modulate adipocyte metabolism. Therefore, the present study aimed to characterize the role of ET-1 in the metabolic consequences of IH on adipose tissue in vivo and in vitro. Wistar rats were submitted to 14 days of IH-cycles (30 s of 21% FiO2 and 30 s of 5% FiO2 ; 8 h day(-1) ) or normoxia (air-air cycles) and were treated or not with bosentan, a dual type A and B endothelin receptor (ETA-R and ETB-R) antagonist. Bosentan treatment decreased plasma free fatty acid and triglyceride levels, and inhibited IH-induced lipolysis in adipose tissue. Moreover, IH induced a 2-fold increase in ET-1 transcription and ETA-R expression in adipose tissue that was reversed by bosentan. In 3T3-L1 adipocytes, ET-1 upregulated its own and its ETA-R transcription and this effect was abolished by bosentan. Moreover, ET-1 induced glycerol release and inhibited insulin-induced glucose uptake. Bosentan and BQ123 inhibited these effects. Bosentan also reversed the ET-1-induced phosphorylation of hormone-sensitive lipase (HSL) on Ser(660) . Finally, ET-1-induced lipolysis and HSL phosphorylation were also observed under hypoxia. Altogether, these data suggest that ET-1 is involved in IH-induced lipolysis in Wistar rats, and that upregulation of ET-1 production and ETA-R expression by ET-1 itself under IH could amplify its effects. Moreover, ET-1-induced lipolysis could be mediated through ETA-R and activation of HSL by Ser(660) phosphorylation.
© 2015 The Authors. The Journal of Physiology © 2015 The Physiological Society.
Figures



Similar articles
-
Endothelin-1 as a novel target for the prevention of metabolic dysfunction with intermittent hypoxia in male participants.Am J Physiol Regul Integr Comp Physiol. 2022 Sep 1;323(3):R351-R362. doi: 10.1152/ajpregu.00301.2021. Epub 2022 Jul 11. Am J Physiol Regul Integr Comp Physiol. 2022. PMID: 35816718 Free PMC article.
-
Fat-reducing effects of dehydroepiandrosterone involve upregulation of ATGL and HSL expression, and stimulation of lipolysis in adipose tissue.Steroids. 2012 Nov;77(13):1359-65. doi: 10.1016/j.steroids.2012.08.002. Epub 2012 Aug 23. Steroids. 2012. PMID: 22951290
-
Endothelin-1 and ET receptors impair left ventricular function by mediated coronary arteries dysfunction in chronic intermittent hypoxia rats.Physiol Rep. 2017 Jan;5(1):e13050. doi: 10.14814/phy2.13050. Physiol Rep. 2017. PMID: 28057852 Free PMC article.
-
Dissecting adipose tissue lipolysis: molecular regulation and implications for metabolic disease.J Mol Endocrinol. 2014 Jun;52(3):R199-222. doi: 10.1530/JME-13-0277. Epub 2014 Feb 27. J Mol Endocrinol. 2014. PMID: 24577718 Review.
-
Endothelin in the pulmonary circulation with special reference to hypoxic pulmonary vasoconstriction.Scand Cardiovasc J Suppl. 1997;46:1-40. Scand Cardiovasc J Suppl. 1997. PMID: 9265559 Review.
Cited by
-
Effect of continuous positive airway pressure on endothelin-1 in patients with obstructive sleep apnea: a meta-analysis.Eur Arch Otorhinolaryngol. 2019 Mar;276(3):623-630. doi: 10.1007/s00405-018-5225-8. Epub 2018 Dec 3. Eur Arch Otorhinolaryngol. 2019. PMID: 30511103
-
Overproduction of endothelin-1 impairs glucose tolerance but does not promote visceral adipose tissue inflammation or limit metabolic adaptations to exercise.Am J Physiol Endocrinol Metab. 2019 Sep 1;317(3):E548-E558. doi: 10.1152/ajpendo.00178.2019. Epub 2019 Jul 16. Am J Physiol Endocrinol Metab. 2019. PMID: 31310581 Free PMC article.
-
Differential Impact of Intermittent vs. Sustained Hypoxia on HIF-1, VEGF and Proliferation of HepG2 Cells.Int J Mol Sci. 2023 Apr 7;24(8):6875. doi: 10.3390/ijms24086875. Int J Mol Sci. 2023. PMID: 37108039 Free PMC article.
-
Chronic intermittent hypoxia, a hallmark of obstructive sleep apnea, promotes 4T1 breast cancer development through endothelin-1 receptors.Sci Rep. 2022 Jul 28;12(1):12916. doi: 10.1038/s41598-022-15541-8. Sci Rep. 2022. PMID: 35902610 Free PMC article.
-
Targeting the ROS-HIF-1-endothelin axis as a therapeutic approach for the treatment of obstructive sleep apnea-related cardiovascular complications.Pharmacol Ther. 2016 Dec;168:1-11. doi: 10.1016/j.pharmthera.2016.07.010. Epub 2016 Aug 2. Pharmacol Ther. 2016. PMID: 27492897 Free PMC article. Review.
References
-
- Anthonsen MW, Ronnstrand L, Wernstedt C, Degerman E & Holm C (1998). Identification of novel phosphorylation sites in hormone‐sensitive lipase that are phosphorylated in response to isoproterenol and govern activation properties in vitro. J Biol Chem 273, 215–221. - PubMed
-
- Arnaud C, Poulain L, Levy P & Dematteis M (2011). Inflammation contributes to the atherogenic role of intermittent hypoxia in apolipoprotein‐E knock out mice. Atherosclerosis 219, 425–431. - PubMed
-
- Belaidi E, Joyeux‐Faure M, Ribuot C, Launois SH, Levy P & Godin‐Ribuot D (2009). Major role for hypoxia inducible factor‐1 and the endothelin system in promoting myocardial infarction and hypertension in an animal model of obstructive sleep apnea. J Am Coll Cardiol 53, 1309–1317. - PubMed
-
- Bhattacharya I & Ullrich A (2006). Endothelin‐1 inhibits adipogenesis: role of phosphorylation of Akt and ERK1/2. FEBS Lett 580, 5765–5771. - PubMed
-
- Campo A, Fruhbeck G, Zulueta JJ, Iriarte J, Seijo LM, Alcaide AB, Galdiz JB & Salvador J (2007). Hyperleptinaemia, respiratory drive and hypercapnic response in obese patients. Eur Respir J 30, 223–231. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

