The effect of LCZ696 (sacubitril/valsartan) on amyloid-β concentrations in cerebrospinal fluid in healthy subjects
- PMID: 26663387
- PMCID: PMC4834603
- DOI: 10.1111/bcp.12861
The effect of LCZ696 (sacubitril/valsartan) on amyloid-β concentrations in cerebrospinal fluid in healthy subjects
Abstract
Aims: LCZ696 (angiotensin receptor neprilysin inhibitor) is a novel drug developed for the treatment of heart failure with reduced ejection fraction. Neprilysin is one of multiple enzymes degrading amyloid-β (Aβ). Its inhibition may increase Aβ levels. The potential exists that treatment of LCZ696, through the inhibition of neprilysin by LBQ657 (an LCZ696 metabolite), may result in accumulation of Aβ. The aim of this study was to assess the blood-brain-barrier penetration of LBQ657 and the potential effects of LCZ696 on cerebrospinal fluid (CSF) concentrations of Aβ isoforms in healthy human volunteers.
Methods: In a double-blind, randomized, parallel group, placebo-controlled study, healthy subjects received once daily LCZ696 (400 mg, n = 21) or placebo (n = 22) for 14 days.
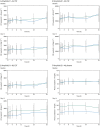
Results: LCZ696 had no significant effect on CSF AUEC(0,36 h) of the aggregable Aβ species 1-42 or 1-40 compared with placebo (estimated treatment ratios 0.98 [95% CI 0.73, 1.34; P = 0.919] and 1.05 [95% CI 0.82, 1.34; P = 0.702], respectively). A 42% increase in CSF AUEC(0,36 h) of soluble Aβ 1-38 was observed (estimated treatment ratio 1.42 [95% CI 1.05, 1.91; P = 0.023]). CSF levels of LBQ657 and CSF Aβ 1-42, 1-40, and 1-38 concentrations were not related (r(2) values 0.022, 0.010, and 0.008, respectively).
Conclusions: LCZ696 did not cause changes in CSF levels of aggregable Aβ isoforms (1-42 and 1-40) compared with placebo, despite achieving CSF concentrations of LBQ657 sufficient to inhibit neprilysin. The clinical relevance of the increase in soluble CSF Aβ 1-38 is currently unknown.
Keywords: CSF; LCZ696; amyloid-β; heart failure; neprilysin.
© 2015 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of The British Pharmacological Society.
Figures



Similar articles
-
The effect of angiotensin receptor neprilysin inhibitor, sacubitril/valsartan, on central nervous system amyloid-β concentrations and clearance in the cynomolgus monkey.Toxicol Appl Pharmacol. 2017 May 15;323:53-65. doi: 10.1016/j.taap.2017.03.014. Epub 2017 Mar 15. Toxicol Appl Pharmacol. 2017. PMID: 28315356
-
Pharmacodynamic and Pharmacokinetic Profiles of Sacubitril/Valsartan (LCZ696) in Patients with Heart Failure and Reduced Ejection Fraction.Cardiovasc Ther. 2016 Aug;34(4):191-8. doi: 10.1111/1755-5922.12183. Cardiovasc Ther. 2016. PMID: 26990595 Free PMC article. Clinical Trial.
-
Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor.J Clin Pharmacol. 2016 Jan;56(1):78-86. doi: 10.1002/jcph.571. Epub 2015 Aug 24. J Clin Pharmacol. 2016. PMID: 26073563 Clinical Trial.
-
Long-term neprilysin inhibition - implications for ARNIs.Nat Rev Cardiol. 2017 Mar;14(3):171-186. doi: 10.1038/nrcardio.2016.200. Epub 2016 Dec 15. Nat Rev Cardiol. 2017. PMID: 27974807 Review.
-
Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition.Circulation. 2016 Mar 15;133(11):1115-24. doi: 10.1161/CIRCULATIONAHA.115.018622. Circulation. 2016. PMID: 26976916 Free PMC article. Review.
Cited by
-
How publication guidelines for clinical pharmacology trials may help to accelerate knowledge transfer.Br J Clin Pharmacol. 2018 Apr;84(4):611-614. doi: 10.1111/bcp.13489. Epub 2018 Feb 9. Br J Clin Pharmacol. 2018. PMID: 29427380 Free PMC article. No abstract available.
-
Updated insights on dementia-related risk of sacubitril/valsartan: A real-world pharmacovigilance analysis.CNS Neurosci Ther. 2023 Sep;29(9):2548-2554. doi: 10.1111/cns.14195. Epub 2023 Mar 27. CNS Neurosci Ther. 2023. PMID: 36971193 Free PMC article.
-
Sacubitril/valsartan in heart failure: efficacy and safety in and outside clinical trials.ESC Heart Fail. 2022 Dec;9(6):3737-3750. doi: 10.1002/ehf2.14097. Epub 2022 Aug 3. ESC Heart Fail. 2022. PMID: 35921043 Free PMC article. Review.
-
Benefits and pitfalls of sacubitril/valsartan treatment in patients with hypertension.J Clin Hypertens (Greenwich). 2018 Feb;20(2):351-355. doi: 10.1111/jch.13169. Epub 2018 Jan 16. J Clin Hypertens (Greenwich). 2018. PMID: 29338112 Free PMC article. No abstract available.
-
Dementia-related adverse events in PARADIGM-HF and other trials in heart failure with reduced ejection fraction.Eur J Heart Fail. 2017 Jan;19(1):129-137. doi: 10.1002/ejhf.687. Epub 2016 Nov 20. Eur J Heart Fail. 2017. PMID: 27868321 Free PMC article. Clinical Trial.
References
-
- Langenickel TH, Dole WP. Angiotensin receptor‐neprilysin inhbition with LCZ696: a novel approach for the treatment of heart failure. Drug Discov Today: Therap Strateg 2012; 9: e131–9.
-
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371: 993–1004. - PubMed
-
- Vardeny O, Miller R, Solomon SD. Combined neprilysin and renin‐angiotensin system inhibition for the treatment of heart failure. JACC Heart Fail 2014; 2: 663–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

